Tag: Gore
Gore reports first clinical use of TAMBE device in Japan
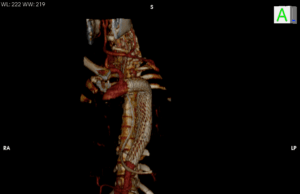
Gore in Japan has announced the first clinical use of the Gore Excluder thoracoabdominal branch endoprosthesis (TAMBE) in the East Asian country. The device,...
ARISE III pivotal study of Gore ascending stent graft for acute...
Gore recently announced the first implantation of the investigational Gore ascending stent graft in the ARISE III trial for the treatment of an acute...
Poll highlights rising concern for radiation exposure among interventionists
A new US-wide poll announced today by Gore and Egg Medical sheds light on the growing concern for radiation exposure in operating rooms and...
Staged open and TEVAR approach with GORE® TAG® Thoracic Branch Endoprosthesis...
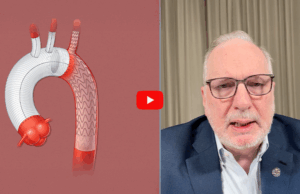
Joseph Bavaria (Jefferson Health, Philadelphia, USA) speaks to Vascular News about a technique he says offers a “complete and total thoracic solution” for acute...
MVSS 2025: Gore iliac branch device “equally effective” in both IDE...
Researchers have reported comparable five-year outcomes of the Gore Excluder iliac branch endoprosthesis (IBE; Gore) in both the investigational device exemption (IDE) and GREAT...
Expanded indication for Gore Viabahn VBX balloon-expandable endoprosthesis as F/BEVAR bridging...
This advertorial, sponsored by Gore, is only available in selected countries and geographies.
The Gore Viabahn VBX balloon-expandable endoprosthesis (VBX stent graft) has achieved...
Gore announces MDR expanded indication for the Viabahn VBX balloon-expandable endoprosthesis...
Gore today announced CE-mark approval of an expanded indication for the Gore Viabahn VBX balloon-expandable endoprosthesis (VBX stent graft) when used as a bridging...
Gore Tag thoracic branch endoprosthesis receives expanded FDA approval for endovascular...
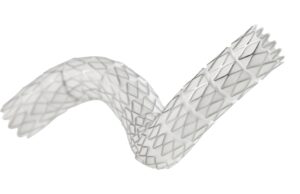
Gore has announced that the Gore Tag thoracic branch endoprosthesis (TBE) is now approved by the US Food and Drug Administration (FDA) for use...
Gore announces US FDA approval and first commercial implant of large-diameter...
Gore recently announced the expansion of the Gore Tag conformable thoracic stent graft with Active Control system product line, following US Food and Drug...
Gore announces first commercial implant of Excluder conformable AAA endoprosthesis with...
Gore recently announced the first commercial use of its Excluder conformable abdominal aortic aneurysm (AAA) endoprosthesis with Active Control system in Canada.
The news coincides...
Gore receives CE mark for lower profile Viabahn VBX endoprosthesis
Gore has announced recent CE mark of a lower profile Viabahn VBX balloon expandable endoprosthesis (VBX stent graft).
Medical Device Regulation (MDR) approval of this...
Vascular News’ top 10 most popular stories of July 2024
Several company updates including Series A funding for Endoron Medical, new insights on the role of machine learning in determining endovascular aneurysm repair (EVAR)...
Gore announces first commercial implant of Gore Tag thoracic branch endoprosthesis...
Gore has announced the first commercial use of the Gore Tag thoracic branch endoprosthesis (TBE) in Canada.
The news came as Canadian government health authority...
Thoraco-aortic surgery set for shift in practice with European launch of...
This advertorial, sponsored by Gore, is only available in selected countries and geographies.
Following the European launch of the recently CE-marked GORE TAG Thoracic...
GORE® TAG® Thoracic Branch Endoprosthesis “meets an area of major need”
This advertorial, sponsored by Gore, is only available in selected countries and geographies.
The GORE TAG Thoracic Branch Endoprosthesis (TBE) recently became the first...
New frontiers in LSA preservation: Dedicated symposium at CX 2024 spotlights...
This advertorial, sponsored by Gore, is only available in selected countries and geographies.
A satellite session at the 2024 Charing Cross (CX) International Symposium...
US FDA approves expanded indication for Gore Excluder conformable device
Gore has announced US Food and Drug Administration (FDA) approval of an expanded indication for the Gore Excluder conformable abdominal aortic aneurysm (AAA) endoprosthesis....
First European implantation of GORE® TAG® Thoracic Branch Endoprosthesis heralds new...
This advertorial, sponsored by Gore, is only available in selected countries and geographies.
“This is a milestone for arch repair,” Dittmar Böckler (Heidelberg, Germany)...
Vascular News’ top 10 most popular stories of February 2024
February's top 10 includes an update from the UK Medicines and Healthcare products Regulatory Agency (MHRA) on the use of paclitaxel-coated devices for peripheral...
Vascular News’ top 10 most popular stories of January 2024
January's top 10 highlights new guidelines from the European Society for Vascular Surgery (ESVS), an expert consensus roundtable on the benefits of intravascular ultrasound...
Type 2 endoleaks in AAA: What is their clinical relevance?
This advertorial, sponsored by Gore, is only available in selected countries and geographies.
Michel Reijnen (Arnhem, The Netherlands) discusses the clinical relevance of type...
Gore announces first European implantation of Gore Tag thoracic branch endoprosthesis
Gore today announced the first ever implantation of the Gore Tag thoracic branch endoprosthesis (TBE) in Europe. The patient was treated by Dittmar Böckler,...
Gore’s Viabahn VBX stent graft receives US FDA approval
Gore has announced recent US Food and Drug Administration (FDA) approval of a lower profile Viabahn VBX balloon expandable endoprosthesis (VBX stent graft).
"Our team...
Gore receives US FDA approval for breakthrough endovascular device in complex...
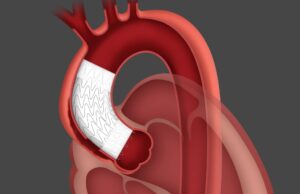
Gore has announced US Food and Drug Administration (FDA) approval for the Gore Excluder thoracoabdominal branch endoprosthesis (TAMBE), which the company states is the...
First patients enrolled in Gore’s VBX FORWARD clinical study
Gore has announced that the first patients have been enrolled in the Gore VBX FORWARD clinical study, a global prospective, multicentre, randomised controlled trial...
First patient treated in ARISE II study of Gore ascending stent...
Gore has announced the first patient implantation of the Gore ascending stent graft in the ARISE II trial, describing this as an exciting step...
“Valuable” 10-year data on the horizon for Gore’s GREAT registry
This advertorial, sponsored by Gore, is only available in selected countries and geographies.
In conversation with CX Vascular, Dennis Gable (The Heart Hospital Baylor...
Vascular News 99 – September 2023
In this issue:
Paclitaxel-coated devices: US FDA removes red flag after review finds data do not support mortality risk
"A hugely exciting time": Experts...
Gore announces clinical study comparing VBX balloon expandable endoprosthesis to bare...
Gore announced today the initiation of the Gore VBX FORWARD clinical study to compare the VBX stent graft to bare metal stenting for patients...
Gore announces first US enrolment for the Viafort vascular stent iliofemoral...
Gore has announced that the first US patient has been enrolled in a prospective, non-randomised, multicentre, single-arm study with five-year follow-up to evaluate the...
Five-year data on GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis “raise the...
This advertorial is sponsored by Gore.
At this year’s Charing Cross (CX) International Symposium (25–27 April, London, UK), Andrew Holden (Auckland City Hospital, Auckland, New...
Patient-centric innovation that stands the test of time
This advertorial is sponsored by Gore.
Even though it is celebrating its aortic portfolio reaching its 25-year milestone, Gore believes that this is only the...
Aortic device conformability: Adapting to the anatomy for better clinical outcomes
This advertorial is sponsored by Gore.
During a satellite symposia at the 2023 Charing Cross (CX) International Symposium (25–27 April, London, UK), speakers addressed the...
First US patient enrolled in Gore Viafort vascular stent pivotal study
W L Gore & Associates (Gore) has announced that the first US patient has been enrolled in a prospective, non-randomised, multicentre, single-arm study with...
Vascular News’ top 10 most popular stories of February 2023
February's top 10 includes the announcement that Abbott is to acquire Cardiovascular Systems, results of the PRESERVE study on the safety and effectiveness of...
Gore initiates EMBRACE registry to evaluate VBX stent graft as bridging...
W L Gore & Associates today announced that it is initiating the EMBRACE registry to capture real-world data about the Gore Viabahn VBX balloon...
Medtronic announces first enrolment in head-to-head global randomised trial evaluating durability...
Medtronic has announced the first patient enrolment in the ADVANCE trial, a head-to-head randomised controlled trial of two leading aortic stent graft systems, the...
Vascular News’ top 10 most popular stories of October 2022
Five-year outcomes from the randomised SPACE-2 trial on carotid artery stenosis, an interview with Joseph S Coselli, and a report on the "significant" increase...
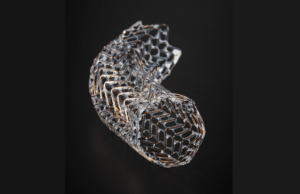
Gore completes first-in-human implants of the Gore Viafort vascular stent
W L Gore & Associates (Gore) today announced the first implants of its investigational Gore Viafort vascular stent as part of the recently initiated...
Gore completes enrolment in the Gore Viabahn VBX balloon-expandable endoprosthesis EXPAND...
W L Gore & Associates (Gore) has announced the completion of target enrolment in the EXPAND postmarket Registry of the Gore Viabahn VBX balloon-expandable...
Vascular News’ top 10 most popular stories of August 2022
The establishment of the first-ever global consensus for the appropriate use of intravascular ultrasound (IVUS) in lower extremity and arterial and venous interventions, results...
Gore acquires InnAVasc Medical
Gore has announced the acquisition of InnAVasc Medical, a privately held medical technology company focused on advancing care for patients with end-stage renal disease...
Gore receives FDA approval of Gore Tag thoracic branch endoprosthesis for...
Gore recently announced that the US Food and Drug Administration (FDA) has approved the Gore Tag thoracic branch endoprosthesis (TBE) for the endovascular repair...
Vascular News’ top 10 most popular stories of March 2022
A review of intraoperative adverse events in patients treated with fenestrated and branched endovascular aneurysm repair, meta-analysis findings on peripheral arterial disease symptoms in...
Viabahn use in claudicants with long, complex SFA lesions “safe and...
Recently published research indicates that stent grafting with the Viabahn endoprosthesis (W L Gore & Associates) of long and complex superficial femoral artery (SFA)...
Five-year results of the Gore Excluder IBE pivotal study confirm safety,...
W L Gore & Associates (Gore) has announced that five-year results from the US prospective, multicentre study (n=63) evaluating endovascular repair of iliac aneurysms...
GORE® ACTIVE CONTROL System: “A system for the masses”
NOTE: This video is ONLY available to watch in selected countries and geographies
Robert Rhee (New York, USA) moderates a Charing Cross 2021 webinar–A...
Real data and experience for below-the-knee bypass back heparin-bonded ePTFE as...
In a Gore-sponsored satellite symposium at the 2021 Charing Cross (CX) Digital Edition (19–22 April, online), Richard Neville (Falls Church, USA) and Walter Dorigo...
Real data and experience for below-the-knee bypass back heparin-bonded ePTFE as...
In a Gore-sponsored satellite symposium at the 2021 Charing Cross (CX) Digital Edition (19–22 April, online), Richard Neville (Falls Church, USA) and Walter Dorigo...
Viabahn balloon-expandable stent can successfully treat occlusive Iliac artery pathology, CX...
In a Gore-sponsored Satellite Symposium at the Charing Cross Symposium (CX; 19–22 April, online), Michele Piazza (Padua, Italy) moderated a discussion on occlusive Iliac...
Great news for EVAR: Registry data shows procedure is safe, durable,...
Endovascular aortic repair (EVAR) is safe, durable, and effective, the five-year interim results of the Global Registry for Endovascular Aortic Treatment (the GREAT registry)...
US physicians begin commercial use of the Gore Excluder conformable AAA...
Today, W L Gore & Associates (Gore) announced the first use of the US Food and Drug Administration (FDA)-approved Gore Excluder conformable abdominal aortic...
FDA approves Gore Excluder conformable AAA endoprosthesis with Active Control system
W L Gore & Associates (Gore) recently announced that the US Food and Drug Administration (FDA) has granted regulatory approval for the new Gore...
Bret Snyder named president and chief executive officer of W L...
W L Gore & Associates recently announced that Bret Snyder, chair of the Gore board of directors, will succeed Jason Field in the role...
Robert W Gore, chairman emeritus of W L Gore & Associates,...
Robert W “Bob” Gore, chair emeritus of the board of directors of W L Gore & Associates, passed away peacefully at home following a...
Gore enhances Viabahn endoprosthesis portfolio with lower profile delivery
Gore has announced the US launch of the lower profile, large diameter Gore Viabahn endoprosthesis.
Gore previously received approval from the US Food and Drug...
Innovative solutions for the treatment of complex aorto-iliac occlusive disease
In this supplement, sponsored by Gore:
Michele Antonello considers the complexity of aorto-iliac occlusive disease, treatment gaps and current options
Jean Bismuth discusses results...
CX 2019: VBX set to be a “game-changer” for treating complex...
𝙏𝙝𝙞𝙨 𝙫𝙞𝙙𝙚𝙤 𝙞𝙨 𝙞𝙣𝙩𝙚𝙣𝙙𝙚𝙙 𝙛𝙤𝙧 𝙝𝙚𝙖𝙡𝙩𝙝𝙘𝙖𝙧𝙚 𝙥𝙧𝙤𝙛𝙚𝙨𝙨𝙞𝙤𝙣𝙖𝙡𝙨 𝙞𝙣 𝙀𝙪𝙧𝙤𝙥𝙚 𝙤𝙣𝙡𝙮.
Giovanni Torsello (Münster, Germany) and Mauro Gargiulo (Bologna, Italy) discuss the “very high” morbidity and...
First US patient receives Gore TAG conformable thoracic stent graft with...
Gore has announced the first US implant of its Gore TAG conformable thoracic stent graft with ACTIVE CONTROL system. The successful procedure was performed...
Gore receives FDA approval for the Gore TAG conformable thoracic stent...
Gore has announced that the US Food and Drug Administration (FDA) has granted regulatory approval for commercial distribution for the Gore TAG Conformable Thoracic...
EVAR with an iliac branch endoprothesis is safe and effective
On Wednesday, during the Aortic Podium 1st session at Charing Cross (CX) Symposium, Fabio Verzini (Turin, Italy) reported that endovascular aneurysm repair (EVAR) with...
Gore Tigris vascular stent demonstrates high patency rates at 12 months
New results suggest that the Tigris vascular stent (Gore) is a safe and effective device that can be incorporated into a modern “leave-nothing-behind” treatment...
Gore introduces GORE TAG Conformable Thoracic Stent Graft with reduced profiles...
Gore has introduced reduced profiles for the most commonly used diameters of the GORE TAG Conformable Thoracic Stent Graft with ACTIVE CONTROL System. The reduced profile...
First patient in Europe receives implant of Gore Excluder Conformable AAA...
Gore has announced the first European patient implant of the Gore Excluder Conformable AAA Endoprosthesis with Active Control System. This next-generation endovascular aneurysm repair...
Gore announces successful patient implant of endovascular stent graft for the...
Gore have announced the first implant in conjunction with the Gore ARISE study of the Gore Ascending Stent Graft, an investigational device and the...
Gore moulding and occlusion balloon for endovascular aortic repair receives approval...
Gore has announced FDA 510(k) clearance, approval from the Japanese Ministry of Health, Labour, and Welfare, and receipt of CE mark for the innovative...
First commercial in-human use of Gore TAG Conformable Thoracic Stent Graft...
The first patient has been implanted with the Gore TAG Conformable Thoracic Stent Graft with Active Control System after being included on the Australian...
Two-year data from Viabahn BX IDE trial “promising”
The 24-month data from the Viabahn BX Flex balloon-expandable stent (Gore) investigational device exemption (IDE) trial are “promising” with sustained clinical and patency benefits...
First patient enrolled in investigational study of the Gore Excluder Conformable...
Gore has announced the first implant of the Gore Excluder Conformable abdominal aortic aneurysms (AAA) endoprosthesis in the USA. The procedure took place on...
First patient enrolled in registry for the Gore TAG Conformable stent...
Gore has announced the first patient enrolment in its post-market European registry for the Gore TAG Conformable Thoracic Stent Graft with Active Control System, following...
First-in-human use TEVAR of Gore TAG thoracic stent graft with Active...
Gore has announced the first patient implant of the TAG conformable thoracic stent graft with Active Control system after the receipt of CE mark...
Excluder Iliac Branch Endoprosthesis device meets mid-term primary endpoints in clinical...
At the 2017 Vascular Annual Meeting (VAM; 30 May–3 June, San Diego, USA), mid-term follow-up data from a pivotal trial (IBE 12-04) of the...
Gore receives regulatory approval for Excluder iliac branch endoprosthesis in Japan
Gore has received Shonin approval from the Japanese Ministry of Health, Labour and Welfare to market the Excluder iliac branch endoprosthesis (IBE), and is...
Tigris stent gets Health Canada approval for peripheral artery disease
Gore has announced the Health Canada approval of the Tigris vascular stent, a dual-component stent with a unique fluoropolymer/nitinol design. The device, which gained...
First implant of Viabahn VBX stent graft completed
Houston Methodist Hospital has become the first US institution to implant the newly FDA-approved Viabahn VBX balloon expandable endoprosthesis (VBX stent graft; Gore), to...
US FDA approves Gore Viabahn expandable stent graft for iliac artery...
The Gore Viabahn VBX balloon expandable endoprosthesis has received US Food & Drug Administration (FDA) approval for treatment of de novo or restenotic lesions found...
Acute Dissections Symposium: “The Great Masquerader”
The best management of type B aortic dissection is an ongoing debate. In April 2016, W. L. Gore & Associates held a two-day symposium...
Gore joins vascular and endovascular leaders in recognising the 2016 Pioneers...
Gore joined with leaders across the vascular and endovascular community in honouring five North American physicians as Pioneers in Performance. The biennial awards programme acknowledges...
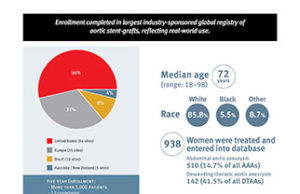
VEITH 2016: Enrolment completed in Gore Global registry
At the 2016 VEITHsymposium (15–19 November, New York, USA), Gore announced that the completion of target enrolment in GREAT (Global registry for endovascular aortic...
TIGRIS trial finds no stent fractures and positive primary patency in...
Twenty-four month TIGRIS trial follow-up data show that treatment using the Tigris nitinol stent (Gore) for long lesions in the superficial femoral and popliteal...
New technology add-on payment awarded for Gore Excluder iliac branch endoprosthesis
The US Centers for Medicare and Medicaid Services (CMS) has granted the Gore Excluder iliac branch endoprosthesis (IBE) new technology status. Beginning on 1...
One-year Japanese results support use of the Viabahn endoprosthesis to treat...
Gore has announced positive results from a prospective, multicentre investigational device exemption (IDE) clinical study of the Viabahn endoprosthesis with heparin bioactive surface in...
First implant completed in pivotal study of Gore TAG thoracic branch...
The first implant of Gore’s TAG thoracic branch endoprosthesis has been completed as part of the device’s pivotal study. The patient was enrolled by...
Iliac branch device results show value of internal iliac artery revascularisation
The new Excluder iliac branch endoprosthesis (Gore) is safe and effective at treating aortoiliac aneurysms and common iliac artery aneurysms, maintaining blood flow into...
Gore Viabahn endoprosthesis celebrates 20 years since introduction
Gore is celebrating the 20th anniversary of the introduction of the Viabahn endoprosthesis; its stent graft for the treatment of complex peripheral vascular disease....
Total endovascular repair of zone 2 aortic arch aneurysms can be...
One-year outcomes of a prospective, non-randomised, feasibility trial evaluating the treatment of aneurysms involving the proximal descending thoracic aorta indicate that total repair can...
Gore DrySeal Flex introducer sheath now commercially available
Gore has announced the commercial availability of the Gore DrySeal Flex introducer sheath, after recently gaining clearance for use by regulatory bodies in the...
Gore Tigris vascular stent gains FDA approval for treatment of peripheral...
Gore has announced US Food and Drug Administration (FDA) approval of the Gore Tigris vascular stent, a dual-component stent with a unique fluoropolymer/nitinol design....
CE mark granted to Gore Excluder conformable AAA prosthesis
Gore has received CE mark for its Excluder conformable abdominal aortic aneurysm (AAA) device, a product designed for the treatment of abdominal aortic aneurysms...