Tag: AVF
Laminate Medical announces first implantation of VasQ AVF device
Laminate Medical has announced the first implantation of their VasQ device in the USA, performed by Ari Kramer (Spartanburg Regional Hospital, Spartanburg, USA). The...
Aperto® DCB: A breakthrough in prolonging dialysis access survival
This advertorial is sponsored by Cardionovum.
Cardionovum’s CE-marked Aperto drug-coated balloon (DCB) is, according to expert opinion and a growing evidence base, spearheading a revolution...
Laminate Medical announces FDA clearance for VasQ AVF creation device
Laminate Medical Technologies has announced their flagship device, the VasQ External Vascular Support, has been cleared by the US Food and Drug Administration (FDA)...
Multi-society guidelines on varicose vein management published
The Society for Vascular Surgery (SVS), American Venous Forum (AVF), and American Vein and Lymphatic Society (AVLS) have released the second and final part...
Enrolment completed in SAVE trial of Selution SLR for AVF treatment
MedAlliance has announced completion of patient enrolment in the SAVE clinical trial with the Selution Sustained Limus Release (SLR) 018 drug-eluting balloon (DEB) for...
WRAPSODY arteriovenous access efficacy (WAVE) pivotal study completes enrolment
Merit Medical has announced that is has completed enrolment in its WRAPSODY arteriovenous access efficacy (WAVE) pivotal study. Merit’s WAVE study is a prospective,...
Alucent Biomedical granted US FDA approval for clinical study
Alucent Biomedical has announced that the US Food and Drug Administration (FDA) granted an investigational device exemption (IDE) for a US clinical study of...
Pathfinder Medical and Imperial College London awarded Innovate UK Smart grant...
Pathfinder Medical has been awarded a Smart grant for a £1.1 million (US$1.4 million) project by Innovate UK, the UK's innovation agency. A press...
The Aperto® drug-coated balloon: A “first-line” treatment for arteriovenous fistula lesions
This advertorial is sponsored by Cardionovum®.
In 2023, there are a number of different options available for treating the vascular access complications of thrombosis and...
Arteriovenous graft use linked to venous thromboembolism risk in haemodialysis patients
In an observational cohort study of Medicare beneficiaries receiving haemodialysis, the use of an arteriovenous graft (AVG) compared with an arteriovenous fistula (AVF) was...
New data bolster safety and effectiveness of Safepax DCB coating technology
This advertorial is sponsored by Cardionovum®. DCB
Matteo Tozzi, associate professor of Vascular Surgery at the University of Insubria (Varese, Italy), speaks to Vascular News...
Intraoperative vascular mapping during haemodialysis access “should be incorporated into routine...
Intraoperative ultrasonographic venous mapping is a useful tool to evaluate vessel suitability for arteriovenous fistula (AVF) creation, Yana Etkin (Zucker School of Medicine at...
VentureMed completes enrolment of Flex Vessel Prep system randomised controlled trial...
VentureMed Group recently announced that it has completed enrolment of a randomised controlled trial (RCT) titled 'Flex Vessel Prep prior to PTA for the...
New sirolimus data provide encouragement despite “potentially disappointing” efficacy results
The results of a small-scale pilot study from Asia have indicated “potentially disappointing” results with a novel drug-eluting balloon in treating dysfunctional arteriovenous access...
Largest real-world experience to date with VasQ device indicates long-term benefits...
The VasQ external support device (Laminate Medical) has demonstrated long-term benefits for radiocephalic (forearm) arteriovenous fistula (AVF) creation in a retrospective analysis of 150...
Humacyte announces publication of positive long-term follow-up data from phase 2...
Humacyte today announced that five-year data from a phase 2 clinical trial of patients receiving the investigational Human Acellular Vessel (HAV) for arteriovenous (AV)...
IMPRESSION trial assessing MagicTouch AVF passes 50% enrolment
Concept Medical has shared the latest update from its IMPRESSION (Sirolimus-coated balloon angioplasty versus plain balloon angioplasty in the treatment of dialysis access dysfunction)...
Dialysis tech firm Pathfinder Medical secures £8.5 million
Pathfinder Medical, which has developed a proprietary technology to guide and accurately align catheters in endovascular procedures allowing clinicians to make connections across blood...
Amplifi™ Vein Dilation System: A novel device under development designed to...
The Amplifi Vein Dilation System (Artio Medical) is is an innovative device designed to dilate arm veins in preparation for AVF surgery using rapid,...
Benefits of percutaneous fistula creation further bolstered by five-year Ellipsys data
The Ellipsys vascular access system (Avenu Medical/Medtronic) can be used to easily and safely create durable percutaneous arteriovenous fistulas (pAVFs) for haemodialysis—with new, long-term...
“Very encouraging” 12-month data from WRAPSODY FIRST study presented at CIRSE...
Twelve-month results from a first-in-human study of the Wrapsody cell-impermeable endoprosthesis (Merit Medical Systems) for the treatment of access circuit stenosis in haemodialysis patients...
Results from first-in-human study of Amplifi vein dilation system presented at...
Preliminary clinical results from a first-in-human study assessing the Amplifi vein dilation system (Artio Medical) were presented at Vascular Interventional Advances (VIVA) 2021 (5–7 October...
Stable incidence and survival of AVFs in Danish dialysis population in...
Despite an older dialysis population, the proportion and survival of arteriovenous fistulas (AVFs) in Danish dialysis patients has not changed over a 39-year period....
Artio Medical completes enrolment of first-in-human trial investigating Amplifi vein dilation...
Artio Medical announced today it has completed enrolment in its first-in-human study evaluating the Amplifi vein dilation system. In the study, five patients were treated...
Oversized balloon angioplasty technique safe and effective for AV fistula maturation
A retrospective analysis published in the Journal of Vascular Access (JVA) has shown that the oversized balloon angioplasty technique to assist arteriovenous (AV) fistula...
Concept Medical provides update on IMPRESSION trial to evaluate MagicTouch AVF
Concept Medical has provided an update on the progress of the IMPRESSION (Sirolimus-coated balloon angioplasty versus plain balloon angioplasty in the treatment of dialysis...
Arteriovenous fistulas contribute to higher survival of haemodialysis patients with COVID-19
A new study, published online in The Journal of Vascular Access (JVA), suggests that arteriovenous fistulas (AVFs) contribute to higher survival of haemodialysis patients...
Vascular Therapies announces clinical results from phase 3 randomised multicentre clinical...
Vascular Therapies recently announced results from its phase 3 clinical trial in which Sirogen showed encouraging arteriovenous fistula (AVF) outcomes in elderly end-stage renal...
Artio Medical announces successful first human use of the Amplifi vein...
Artio Medical recently announced that it has successfully completed the first human use of its Amplifi vein dilation system. The first clinical procedure was...
Arterial diameter may help to predict AVF aneurysm progression, study suggests
A recent study concludes that arterial diameter may influence arteriovenous fistula (AVF) aneurysm progression and the interval to surgical revision. “Patients with larger arterial...
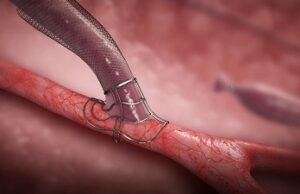
VasQ device improves AVF creation in haemodialysis patients
VasQ, a high haemocompatibility biosynthetic vascular device from Laminate Medical Technologies, could be protective against the haemodynamic modifications that occur during arteriovenous fistulae (AVF)...
Alucent Biomedical adds to scientific advisory board, expands portfolio with AF...
Alucent Biomedical has announced Stanford vascular surgeon Venita Chandra (Stanford University Medical Center, Stanford, USA) will join the company’s scientific advisory board. The news...
Patient-specific computation model may improve AVF maturation rates
The results of a randomised controlled trial—the Shunt simulation study—show that a new patient-specific computation model accurately calculated postoperative access flow. As flow is...
Artio Medical acquires Flow Forward Medical, expanding their peripheral vascular portfolio
Artio Medical today announced it has acquired Flow Forward Medical, a medical device company developing methods for establishing and maintaining vascular access sites.
This...
APERTO becomes the first drug-coated balloon to be approved in China...
APERTO, Cardionovum’s paclitaxel-coated balloon for arterio-venous access, has received market approval for China, making it the first high pressure drug-coated balloon (DCB) available in...
VIVA 2019: IN.PACT AV DCB shows “consistent effectiveness performance” in subgroup...
Today Medtronic shared a six-month subgroup analysis from the IN.PACT AV Access trial, which evaluated how the IN.PACT AV DCB—an investigational device not currently...
Comparing and contrasting surgically-created AVFs with those made by endovascular means
Nicholas Inston (Birmingham, UK) tells Vascular News at LINC 2019 about the exciting field of percutaneous arteriovenous fistula (AVF) creation, the main devices and...
Flow Forward Medical exceeds US$8 million investments, awarded grant from NIH
Flow Forward Medical, a medical device company focused on improving outcomes for haemodialysis patients through the rapid creation of high-quality vascular access sites, has...
Meta-analysis shows positive experience for endoAVF patients for haemodialysis access
An international meta-analysis of clinical experience in patients who received an endovascular arteriovenous fistula (endoAVF) for haemodialysis access was presented at Leipzig Interventional Course...
TVA Medical receives CE mark for everlinQ 4 endovascular AVF system
TVA Medical’s everlinQ 4 endovascular arteriovenous fistula (AVF) system has received CE mark in the European Union. The technology uses a 4F catheter system...
Twelve-month NEAT trial data promising for everlinQ endoAVF haemodialysis access system
TVA Medical has announced the online publication of positive results from the Novel Endovascular Access Trial (NEAT) evaluating its everlinQ endoAVF system. The 12-month...
Bluegrass Vascular announces strategic relationship with Merit Medical
Bluegrass Vascular Technologies is entering into a strategic relationship with Merit Medical Systems. The agreement will streamline European distribution of the recently CE mark-approved Surfacer Inside-Out...
Promising early results for Lutonix US IDE trial in obstructed AV...
The first US randomised controlled trial for drug-coated balloon use for arteriovenous fistula obstructions for 50 years, the Lutonix (Bard) IDE trial was a...
VasQ series at Berlin’s Charité University Hospital produces “exceptionally promising results”
As part of the main scientific programme at Dreiländertagung 2016 (4–8 October, Bern Switzerland), Peter Olschewski has reported “exceptionally promising results” from the first...