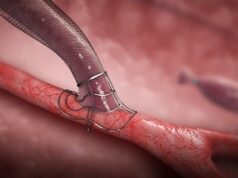
The US Food and Drug Administration (FDA) has designated Laminate Medical’s VasQ external support for the creation of arteriovenous fistulas (AVF) in haemodialysis patients as a breakthrough device.
According to a press release, the designation was based in part on the breadth of clinical evidence collected so far for VasQ that consistently demonstrates improvement over the standard of care for creating functional AVFs for haemodialysis treatment.
“I have been anxious to get my hands back on VasQ ever since I reached the maximum enrolment for a site in the US clinical study,” said Jason Burgess of the Surgical Specialists of Charlotte (Charlotte, USA). “I am excited that the FDA has recognised the urgency we have as surgeons to improve the woeful outcomes that plague fistula creation.”
VasQ is currently in a US investigational device exemption (IDE) pivotal clinical study that is expected to have completed follow-up for its primary endpoints by August 2020 and will be evaluated by the FDA through the de novo pathway.
As of 4 February 2020, the single-arm study has prospectively enrolled its entire 144 male and female patient cohort from 15 sites across the USA. Both current and pre-dialysis patients referred for fistula creation where included in the study for either a brachiocephalic or radiocephalic fistulas with VasQ.
The patients will be followed for two years with the primary efficacy endpoint of unassisted primary patency to be analysed at six months. The FDA will consider the results from the study as well as the breadth of clinical evidence collected from use of the device around the world in its decision to grant US market clearance.
“This is a significant milestone for VasQ that will enable a faster and smoother regulatory process for the US market as well as support the necessary reimbursement for the use of the device in clinical practice to benefit patients,” stated Orit Yarden, vice president of Clinical and Regulatory Affairs for Laminate.