Tag: shockwave medical
Shockwave Medical announces US launch of intravascular lithotripsy catheter
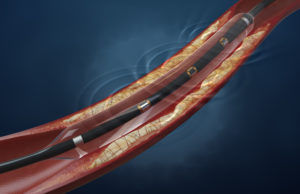
Today, Shockwave Medical announced the US launch of its Shockwave Javelin peripheral intravascular lithotripsy (IVL) catheter, a platform designed to modify calcium and cross...
Vascular News’ top 10 most popular stories of November 2024
Late-breaking data shared at VIVA 2024 (3–6 November, Las Vegas, USA), the first-in-human implantation of a new bridging stent, and an interview with Ramon Varcoe...
Treatment of the common iliac arteries with Shockwave L6: Preserving future...
This article forms part of an educational supplement sponsored by Shockwave Medical. Explore the full Shockwave L6 series here.
Ashish Patel (Guy’s and St Thomas’ NHS Foundation...
Changing the treatment mindset for calcified external iliac artery disease
This article forms part of an educational supplement sponsored by Shockwave Medical. Explore the full Shockwave L6 series here.
In this case report, Stefano Fazzini (University of...
Do we need to stent every calcified iliac lesion?
This article forms part of an educational supplement sponsored by Shockwave Medical. Explore the full Shockwave L6 series here.
Mark Portou (Royal Free Hospital, London, UK) presents...
A paradigm shift in the management of severe iliac calcification
This article forms part of an educational supplement sponsored by Shockwave Medical. Explore the full Shockwave L6 series here.
Charting the history of treatment for severely calcified...
Is it time to rethink your treatment strategy in calcified iliac...
This educational supplement is sponsored by Shockwave Medical. Explore the full Shockwave L6 series here.
In this supplement:
Michel Bosiers (University Hospital Bern, Bern, Switzerland)...
Shockwave Javelin peripheral IVL catheter meets prespecified efficacy and safety performance...
Shockwave Medical, part of Johnson & Johnson MedTech, has announced the first clinical outcomes associated with the Shockwave Javelin peripheral intravascular lithotripsy (IVL) catheter,...
Vascular News’ top 10 most popular stories of September 2024
Six-month data from Merit Medical's Wrapsody WAVE trial, an update on the NHS England Acute Aortic Dissection Toolkit, and a large meta-analysis of endovascular...
Shockwave Medical expands US peripheral IVL portfolio with enhanced catheter
Shockwave Medical, part of Johnson & Johnson MedTech, has announced the full US launch of its Shockwave E8 peripheral intravascular lithotripsy (IVL) catheter, following...
Johnson & Johnson completes acquisition of Shockwave Medical
Johnson & Johnson today announced it has completed its acquisition of Shockwave Medical. Shockwave is now part of Johnson & Johnson and will operate...
Vascular News 99 – September 2023 US Edition
In this issue:
Paclitaxel-coated devices: US FDA removes red flag after review finds data do not support mortality risk
“A hugely exciting time”: Experts...
Shockwave L6 catheter expands treatment options for large-vessel cases
NOTE: This video is ONLY available to watch in selected countries and geographies
The benefits of Shockwave Medical’s new Shockwave L6 Peripheral Intravascular Lithotripsy (IVL)...
Shockwave L6 catheter achieves ‘almost pristine’ result in challenging case
NOTE: This video is ONLY available to watch in selected countries and geographies
Experts gathered at the Society for Vascular Surgery’s 2023 Vascular Annual...
Adding 8–12mm diameter devices to the Shockwave Peripheral Intravascular Lithotripsy toolkit
This advertorial is sponsored by Shockwave Medical.
Mazin Foteh, MD, contrasts the benefits of Shockwave Medical’s new Shockwave L6 Peripheral Intravascular Lithotripsy Catheter alongside those...
Vascular News’ top 10 most popular stories of March 2023
The US launch of a new peripheral intravascular lithotripsy (IVL) catheter, new data on large bore mechanical thrombectomy in high-risk pulmonary embolism (PE) patients,...
Shockwave Medical announces US launch of new peripheral IVL catheter
Shockwave Medical today announced the full US commercial availability of the Shockwave L6 peripheral intravascular lithotripsy (IVL) catheter following clearance by the US Food...
Shockwave IVL the “final piece of the puzzle” to safe, effective...
As the new kid on the block, endovascular treatment of the common femoral artery (CFA) just does not have that long-term outcome data yet,”...
Choose the right endograft for your patient with Shockwave IVL
Giacomo Isernia (Perugia, Italy) sits down with Vascular News to discuss the challenges posed by calcium when it comes to endograft delivery. Calcium “limits the ability...
The advantages of an endovascular approach for CFA treatment with Shockwave...
Treatment of common femoral artery (CFA) stenosis, either with an open or endovascular approach, is the subject of much debate, Raphaël Coscas (Paris, France)...
Shockwave IVL can preserve patients’ future options by increasing vessel wall...
Bella Huasen (Preston, UK) talks to Vascular News about some of the difficulties of treating occlusive disease in the femoropopliteal segment and why it is “essential...
Shockwave IVL: A paradigm shift in the treatment of common femoral...
This article forms part of an educational supplement sponsored by Shockwave Medical. Explore the full Compliance is Key series here.
Raphaël Coscas (Ambroise Paré Hospital, AP-HP...
Shockwave IVL: The last piece of the puzzle to overcome the...
This article forms part of an educational supplement sponsored by Shockwave Medical. Explore the full Compliance is Key series here.
Giacomo Isernia and Gioele Simonte...
Shockwave IVL: Improving compliance is the key to “best endovascular” CFA...
This article forms part of an educational supplement sponsored by Shockwave Medical. Explore the full Compliance is Key series here.
In this case report, Narayanan...
Shockwave IVL: Preserving options in no-stent zones
This article forms part of an educational supplement sponsored by Shockwave Medical. Explore the full Compliance is Key series here.
Bella Huasen (Lancashire Teaching Hospitals...
Shockwave IVL: Sound science reinforced by sound evidence
This article forms part of an educational supplement sponsored by Shockwave Medical. Explore the full Compliance is Key series here.
Stefano Fazzini (Tor Vergata Hospital,...
Change compliance to change the game in PAD treatment: Overcome the...
This educational supplement is sponsored by Shockwave Medical. Explore the full Compliance is Key series here.
In this supplement:
Stefano Fazzini (Tor Vergata Hospital, Rome,...
Vascular News’ top 10 most popular stories of November 2022
November's top 10 includes the first results from BEST-CLI, as well as new data presented at this year's Vascular Interventional Advances (VIVA; 31 October–3...
Disrupt PAD III observational study results confirm safety and effectiveness of...
The final 1,373-patient cohort analysis from the Disrupt PAD III observational study (OS) demonstrates consistent intravascular lithotripsy (IVL; Shockwave Medical) outcomes in complex and...
Shockwave IVL: Maintaining options for patients with calcified PAD
Intravascular Lithotripsy (IVL; Shockwave Medical) gives physicians “the option to treat a patient efficiently without implanting something like a stent, which would limit further...
Shockwave IVL: Seeing is believing
Raphaël Coscas (Paris, France) talks to Vascular News about his introduction to Intravascular Lithotripsy (IVL; Shockwave Medical) and his initial experiences with the technology in...
Vascular News’ top 10 most popular stories of May 2022
Two-year data on the use of intravascular lithotripsy (IVL) in calcified peripheral disease treatment, an advertorial sponsored by Bentley exploring the role of bridging...
Shockwave Medical and Genesis MedTech obtain regulatory approval in China for...
Shockwave Medical and Genesis MedTech Group announced today that they have successfully obtained approval from China’s National Medical Products Administration (NMPA) to market and...
Shockwave IVL maintains superiority to angioplasty in calcified peripheral disease at...
Shockwave Medical announced today that long-term data from the Disrupt PAD III trial found that superior vessel preparation with intravascular lithotripsy (IVL) led to...
Confined by the complexity of calcium? Expand the boundaries with Shockwave...
This educational supplement is only available in selected countries and geographies.
In this supplement, sponsored by Shockwave Medical:
Marianne Brodmann (Medical University of...
Shockwave Medical announces global launch of new peripheral IVL catheter
Shockwave Medical has announced the global commercial availability of the Shockwave M5+ peripheral intravascular lithotripsy (IVL) catheter after receiving both CE mark and US...
Vascular News’ top 10 most popular stories of November 2021
November's top 10 features a large-scale analysis of Philips' intravascular ultrasound (IVUS) in lower extremity peripheral vascular interventions, five-year data on Avenu Medical/Medtronic's Ellipsys...
Shockwave Medical enrols first patient in Disrupt BTK II study for...
Shockwave Medical has announced the start of the Disrupt BTK II postmarket study to assess the safety, effectiveness and optimal clinical use of the...
CMS increases hospital outpatient payment for peripheral intravascular lithotripsy
Shockwave Medical has announced that, as part of the calendar year 2022 Medicare Hospital Outpatient Prospective Payment System (OPPS) final rule, the Centers for...
VIVA 2021: IVL “consistently” treats real-world calcium in multiple peripheral vessel...
An interim analysis from the DISRUPT PAD III observational study showed that intravascular lithotripsy (IVL; Shockwave Medical) performs “consistently well” across challenging peripheral vessels,...
CMS creates new codes that establish specific payment for IVL performed...
Shockwave Medical has announced that as part of the calendar year 2021 Medicare Hospital Outpatient Prospective Payment System (OPPS) final rule, the Centers for...
The emerging value and broad applicability of intravascular lithotripsy
In this supplement, sponsored by Shockwave:
Frank Arko discusses his experience with intravascular lithotripsy (IVL) and considers its value in a resource-constrained healthcare environment
...
Shockwave Medical announces that CMS has created new codes for intravascular...
Shockwave Medical announced today that the Centers for Medicare & Medicaid Services (CMS) has issued new codes for intravascular lithotripsy (IVL) procedures performed in...
Shockwave IVL can aid in eliminating recoil and dissection during below-the-knee...
George Adams (Raleigh, USA) talks to VEITHtv at the VEITHsymposium 2019 (19–23 November, New York, USA) about the benefits of using easy-to-use Shockwave IVL (Intravascular Lithotripsy) in calcified...
IVL has “changed the dynamics” of facilitating EVAR & TEVAR in...
Frank Arko (Charlotte, USA) talks to VEITHtv at the VEITHsymposium 2019 (19–23 November, New York, USA), about the benefits of using Shockwave Intravascular Lithotripsy (IVL) when performing EVAR...
Shockwave announces commercial availability of below-the-knew IVL catheter
Shockwave Medical today announced commercial availability of the Shockwave S4 peripheral intravascular lithotripsy (IVL) catheter in select approved geographies. Shockwave S4 is a low-profile...
Intra-arterial calcification poses “one of the biggest challenges” for endovascular therapy
Andrew Holden (Auckland, New Zealand) provides perspective on the first results from the Disrupt PAD III observational registry that were presented at the Charing...
Abiomed invests $15 million in Shockwave Medical
Abiomed is to invest US$15 million in Shockwave Medical and the two companies will collaborate on a training and education programme in the USA...
Consider intravascular lithotripsy when treating calcified femoropopliteal arteries
Treating severely calcified arteries by endovascular means is still a challenge, although atherectomy, stents and lithotripsy are all techniques that are employed. Lithotripsy (also...
Novel approach in treating stenotic peripheral arteries shown to be safe...
Twelve-month data prove that intravascular lithotripsy; a novel approach using pulsatile sonic pressure waves to modify intimal and medium calcium in stenotic peripheral arteries,...
“Fantastic” live case demonstrates potential value of intravascular lithotripsy
In an exciting session at the Charing Cross Symposium (CX; 24-27 April, London, UK), the audience witnessed the demonstration of a novel treatment strategy...
Shockwave Medical announces US$35 million in new financing
Shockwave Medical has reported US$35 million in new financing, an extension of the company’s previously announced US$45 million Series C financing. New investor Fidelity Management...
Shockwave Medical reports positive results for Lithoplasty in calcified lesions below...
Shockwave Medical has reported positive results from the DISRUPT BTK Study, which were presented at the annual Cardiovascular and Interventional Radiological Society of Europe...
Shockwave Medical Lithoplasty system launched in USA as first patient enrolled...
Shockwave Medical has announced two milestones for its Lithoplasty system for the treatment of calcified plaque in patients with peripheral artery disease (PAD): enrolment...
Shockwave Medical appoints Doug Godshall as president and chief executive officer
Shockwave Medical has announced the appointment of Doug Godshall as president and chief executive officer.
Godshall was most recently the chief executive officer and a...
First US patient treated in vascular study of Shockwave Medical Lithoplasty...
PinnacleHealth CardioVascular Institute has enrolled the first US patient in a trial assessing the safety and effectiveness of a new type of approach for...
Shockwave Medical raises US$45 Million in Series C financing to advance...
Shockwave Medical has announced the closing of US$45 million in Series C financing led by Sectoral Asset Management, with participation from mutual funds advised...
Shockwave Medical announces plans for “largest ever” calcified peripheral artery disease...
Shockwave Medical has announced plans for DISRUPT PAD III—what is claims is the “largest ever” multicentre randomised study to exclusively enrol patients with calcified...
Six-month Shockwave Medical Disrupt PAD data show “consistent and predictable” procedural...
Shockwave Medical has announced positive clinical results from the pooled DISRUPT PAD Study, a single-arm, two-phase, multicentre study evaluating the safety and performance of...
Shockwave Medical Lithoplasty system is cleared by the FDA
Shockwave Medica has announced clearance from the US Food and Drug Administration (FDA) of the Lithoplasty system for the treatment of calcified plaque in...
CE mark approval for Lithoplasty balloon catheters for the treatment of...
Lithoplasty is a novel balloon-based technology that utilises integrated lithotripsy, a pulsatile mechanical energy commonly used to break up kidney stones, to disrupt both superficial and deep calcium and normalise vessel wall compliance prior to low-pressure balloon dilatation.