Endologix has announced that the CE mark for the Nellix endovascular aneurysm sealing (EVAS) system has been reinstated. The CE mark for the system was suspended on 22 January and now it that has been reinstated, Nellix will be made available for use at approved centres in a post-market clinical investigational setting outside the USA. Within the USA, Nellix continues to be an investigational device as part of the EVARS2 study.
A few weeks prior to the suspension of the CE mark, on 4 January, Endologix released a statement that it was taking “decisive action to optimise patient outcomes” with the Nellix system. In the statement, the company reported that unrestricted sales and use of the system would “cease immediately” and that Nellix would “only be available for use under clinical protocol with pre-screened patients that adhere to the current indications”. Further to the statement, Endologix voluntarily recalled all existing inventory and issued a field safety notification. Matt Thompson, chief medical officer of Endologix, said at the time: “We monitor the performance of the Nellix system through clinical trials, our complaint monitoring system, physician interaction and available publications. Our independently adjudicated data from the EVAS1 IDE clinical trial indicates that the Nellix System has performed well when used consistently with the current indications. However, data from recent Nellix publications leave us concerned that outcomes are suboptimal when the system is used outside current instructions for use.”
Last year, in the European Journal of Vascular Endovascular Surgery, Seamus C Harrison (Addenbrooke’s Hospital, Cambridge Hospitals University Trust, Cambridge, UK) and colleagues reported outcomes for patients who underwent EVAS with the Nellix system at their centre (Cambridge University Hospitals Foundation Trust). They found that the system was associated with “poor outcomes” beyond the first two years following implantation, noting that “of the patients surviving more than three years, approximately half of the grafts have shown signs of failure”. The authors add: “Diagnosis and management of proximal graft failure is challenging and often requires graft explant. Nellix is no longer routinely used at Cambridge University Hospital because of these problems.”
However, in this study, 29% of patients (161 overall) had anatomy outside the original instructions for use (IFU) for Nellix and another 25% had no other endovascular option (including branched or fenestrated endovascular aneurysm repair). The Endologix statement announcing the voluntary recall decrees that off-label use of Nellix has been occurring at “an unacceptable level, with the consequence of suboptimal results”. Furthermore, in the statement, John Onopchenko, chief executive officer of Endologix, comments: “Ensuring patient safety and optimal clinical outcomes is our top priority, and the current level of off-label use of the Nellix system cannot continue if we are to protect and preserve the potential for transformative EVAS therapy. Taking these actions aligns with clinical practice standards, allows us to control off-label use, and will help us ensure appropriate application of the therapy.”
Nellix was designed to improve the long-term durability of endovascular aneurysm repair (EVAR) by reducing the incidence of endoleaks (all types). Harrison et al report: “The system is based upon two balloon expandable stents attached to ‘endobags’ that are filled in situ with a soluble polymer that ‘seals’ the aneurysm”. They add that, when first introduced, Nellix had anatomical IFU that “were more liberal than other stent graft manufacturers, thereby increasing the applicability of minimally invasive repair”. Therefore, now that its CE mark has been reinstated, Nellix will be—albeit in a limited way—on the EVAR market.
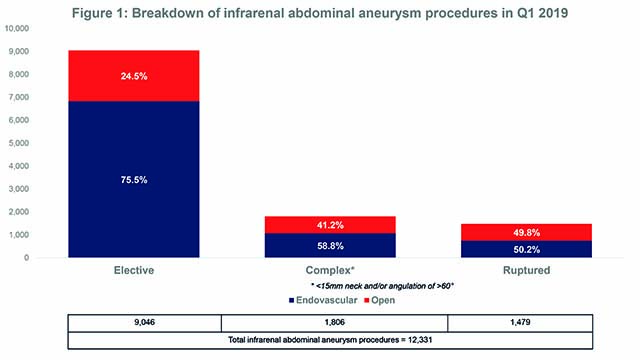
Infrarenal abdominal aneurysm market in Western Europe

According to the BIBA MedTech Aortic Segmentation Monitor, 59% of all aortic procedures (20,932 overall) performed in Q1 (1 January–31 March) were for infrarenal abdominal aneurysms. Of these, 70% were performed with an endovascular approach. Elective repair was the most common indication for an infrarenal abdominal aneurysm procedure (of elective repairs, 75.5% were endovascular). See Figure 1.
In the overall infrarenal market, in Q1 2019, 8,622 devices were used. Medtronic was the market leader with a market share of 42.8%. Of note, Endologix (with its AFX and Ovation ranges) had a market share of 4.9%. See Figure 2.
How the CE mark for Nellix being reinstated will affect the Western European infrarenal abdominal aneurysm market is unknown at present, but Endologix has claimed that it expects neither the suspension nor the reinstatement to “impact its previously communicated guidance for 2019”. In May 2019, Endologix reported that its global revenue for Q1 2019 was US$35.6m—a 15.8% decrease from Q1 2018 (which was US$42.3m). The company added, reaffirming its annual guidance, that it expects to have revenue of at least US140m this year. Onopchenko comments: “Our first quarter financial and operational performance provides us with a solid foundation to accomplish our goals for the rest of 2019, as we continue to execute against our strategic plan and regain credibility and traction in the marketplace. We recently addressed the vast majority of our near-term debt maturities while increasing our liquidity position, allowing us to move forward with a singular focus on maintaining consistent delivery against our commitments. Those commitments include producing compelling evidence that demonstrates improved patient outcomes and supports the introduction of innovative products. I am very proud of what the team has accomplished thus far in 2019, and I strongly believe our evolving culture of accountability will allow us to maintain this level of focus and performance going forward.”
This article is part of a series of BIBA Briefings columns in Vascular News. For previous columns, see past issues.
BIBA Briefings Insights reports give in-depth analysis of the latest market intelligence from BIBA MedTech Insights. They also review that the latest technology news and pipeline developments.
For editorial enquiries, please contact Dawn Powell: [email protected]
For sales enquiries (including BIBA MedTech Insights), please contact Merveille Anderson:[email protected]












