Tag: paclitaxel
Drug-eluting technologies should be ‘de facto standard of care’ for PAD,...
New data from a large, real-world study support the use of drug-eluting devices to reduce amputations, readmissions, and healthcare costs in the treatment of...
Drug wars, resorbables and extensive calcification: Maryanne Brodmann delivers a broadside...
Marianne Brodmann (Medical University of Graz, Graz, Austria) shared her opinions on some of the burning debates in peripheral arterial disease (PAD) treatment at...
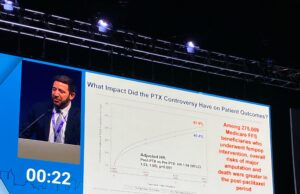
Study finds adverse outcomes after decreased use of paclitaxel-coated devices
A recent analysis of over 270,000 Medicare fee-for-service beneficiaries has found an increase in adverse outcomes and death after a US Food and Drug...
New vessel preparation and drug delivery data spotlighted at LINC 2024
Sabine Steiner (University of Leipzig, Leipzig, Germany) took to the podium at this year’s Leipzig Interventional Course (LINC 2024; 28–31 May, Leipzig, Germany) to...
CX 2024: Paclitaxel device restrictions “did cause harm”
“Unfortunately, we are doing worse for our patients today,” were the sobering thoughts of Eric Secemsky (Boston, United States) during a late-breaking presentation in...
New data and heated debates set to spark controversy in CX...
“These will be important, salutary lessons. Should a controversy arise again involving a proven efficacious therapy, we now know that stopping access to that...
Vascular News’ top 10 most popular stories of February 2024
February's top 10 includes an update from the UK Medicines and Healthcare products Regulatory Agency (MHRA) on the use of paclitaxel-coated devices for peripheral...
TRANSCEND 36-month data “continue to demonstrate safe and effective performance” of...
Peter Schneider (University of California San Francisco, San Francisco, USA) recently presented 36-month data from Surmodics' TRANSCEND clinical trial at the VEITHsymposium 2023 (14–18...
TCT 2023: First presentation of complete patient-level dataset on paclitaxel and...
Data from a patient-level meta-analysis—a factor in the US Food and Drug Administration’s (FDA) recent change of position on the use of paclitaxel-coated devices...
Vascular News 99 – September 2023 US Edition
In this issue:
Paclitaxel-coated devices: US FDA removes red flag after review finds data do not support mortality risk
“A hugely exciting time”: Experts...
Vascular News 99 – September 2023
In this issue:
Paclitaxel-coated devices: US FDA removes red flag after review finds data do not support mortality risk
"A hugely exciting time": Experts...
FDA update: Paclitaxel-coated devices for PAD cleared of excess mortality risk,...
This advertorial is sponsored by Boston Scientific.
Data on hundreds of thousands of patients who have received lower limb endovascular treatment provide confidence in paclitaxel...
Vascular News’ top 10 most popular stories of July 2023
July's top 10 highlights the US Food and Drug Administration's long-awaited update on paclitaxel-coated devices to treat peripheral arterial disease, an interview with Rachel...
SCAI welcomes FDA decision to remove red flag for use of...
The president of the Society for Cardiovascular Angiography & Interventions (SCAI), George Dangas (Icahn School of Medicine at Mount Sinai, New York, USA), has...
CIRSE supports use of paclitaxel-coated devices in femoropopliteal disease treatment
In June 2023, the Cardiovascular Interventional Radiological Society of Europe (CIRSE) published an editorial in Cardiovascular Interventional Radiology (CVIR). Written on behalf of CIRSE’s...
Timeline: Key milestones in the paclitaxel story
The release of a letter from the US Food and Drug Administration (FDA) concerning the use of paclitaxel-coated devices for the peripheral arterial disease...
Boston Scientific announces position on FDA update about use of paclitaxel-coated...
Following yesterday's news that the US Food and Drug Administration (FDA) has changed its stance on the use of paclitaxel-coated devices to treat peripheral...
FDA removes red flag for paclitaxel-coated devices after review finds data...
In a letter to healthcare providers dated 11 July 2023, the US Food and Drug Administration (FDA) communicates that the risk of mortality associated...
Real-world data consistent with RCTs in highlighting the efficacy of drug-eluting...
This advertorial is sponsored by Boston Scientific.
During a recent satellite symposium, which took place at the 2022 Cardiovascular Interventional Radiological Society of Europe (CIRSE)...
Concept Medical’s SIRONA trial completes enrolment
Concept Medical has announced the completion of patient enrolment in the SIRONA trial. According to the company, this is the world's first and the...
First US patient enrolled in Selution SLR IDE peripheral study
The first US patient has been enrolled in the US Food and Drug Administration (FDA) SELUTION4BTK (below-the-knee) clinical trial evaluating Selution SLR, MedAlliance's novel...
Vascular News’ top 10 most popular stories of May 2022
Two-year data on the use of intravascular lithotripsy (IVL) in calcified peripheral disease treatment, an advertorial sponsored by Bentley exploring the role of bridging...
New long-term data of paclitaxel devices continue to show no increased...
New long-term data from the SAFE-PAD (Safety assessment of femoropopliteal endovascular treatment with paclitaxel-coated devices) study were presented today as late-breaking clinical research at...
Five-year patient-level data confirm safety of Passeo-18 Lux paclitaxel DCB
New long-term data presented at the 2022 Charing Cross (CX) Symposium (26–28 April, London, UK) demonstrate the safety of Biotronik’s Passeo-18 Lux paclitaxel drug-coated...
Medtronic issues voluntary recall for subset of IN.PACT Admiral and IN.PACT...
Medtronic recently voluntarily recalled a subset of its IN.PACT Admiral and IN.PACT AV paclitaxel-coated percutaneous transluminal angioplasty (PTA) balloon catheters due to the potential...
SIRONA head-to-head randomised trial achieves 50% enrolment
Concept Medical recently announced that the SIRONA randomised controlled trial (RCT)—a head-to-head comparison of sirolimus versus paclitaxel drug-eluting balloon angioplasty in the femoropopliteal artery—has...
BIOPACT head-to-head non-inferiority randomised controlled trial completes enrolment
Biotronik is proud to announce the completion of enrolment of the investigator-initiated BIOPACT randomised controlled trial (RCT). This non-inferiority study evaluates the safety and...
Medtronic receives CE mark for 200mm and 250mm IN.PACT Admiral DCBs
Medtronic has announced CE mark approval and the European launch of the 200mm and 250mm IN.PACT Admiral drug-coated balloons (DCBs). The product is intended...
Vascular News’ top 10 most popular stories of July 2021
Updated Society for Vascular Surgery (SVS) clinical practice guidelines on extracranial cerebrovascular disease management and a new meta-analysis on paclitaxel in peripheral arterial disease...
Latest meta-analysis on paclitaxel in peripheral arterial disease provokes mixed reception
“There appears to be heightened risk of major amputation after use of paclitaxel-coated balloons in the peripheral arteries,” findings from a systematic review...
Nationwide study identifies sex disparities in long-term mortality after paclitaxel exposure
A German claims-based cohort study has revealed that—in 13,204 patients treated with a paclitaxel-coated device for peripheral arterial disease (PAD)—mortality differences were mostly attributable...
Concept Medical releases status updates on SIRONA RCT
Concept Medical has released a series of status updates on their head-to-head SIRONA (Sirolimus versus paclitaxel drug-eluting balloon angioplasty in femoropopliteal diseases) randomised controlled...
ACC.21: SAFE-PAD finds no increased risk of death with drug-coated devices...
Researchers have found no statistically significant difference in mortality between patients treated with drug-coated devices and non-drug-coated devices in the SAFE-PAD study. Eric Secemsky...
Vascular News’ top 10 most popular stories of April 2021
Highlights from the Charing Cross (CX) 2021 Digital Edition (19–22 April, online)—including the potential of Philips’ Fiber Optic RealShape (FORS) technology to reduce radiation...
CX audience supports call to change agency recommendations regarding paclitaxel use...
Addressing representatives from the US Food and Drug Administration (FDA) and UK Medicines and Healthcare products Regulatory Agency (MHRA) directly, Thomas Zeller (Bad Krozingen,...
CX audience supports call to change agency recommendations regarding paclitaxel use...
Addressing representatives from the US Food and Drug Administration (FDA) and UK Medicines and Healthcare products Regulatory Agency (MHRA) directly, Thomas Zeller (Bad Krozingen,...
CX to host “vital” global discussion of paclitaxel controversy
Andrew Holden (Auckland City Hospital; University of Auckland, Auckland, New Zealand) speaks to Vascular News ahead of this year’s Charing Cross Symposium (CX), which...
LINC 2021: Head-to-head trials take centre stage in drug-eluting technology late-breaking...
Data from two head-to-head trials on drug-eluting technologies in femoral artery treatment were presented during a late-breaking session at LINC 2021 (The Leipzig Interventional...
SWEDEPAD unplanned interim analysis shows no difference in all-cause mortality for...
An unplanned interim analysis of the registry-based SWEDEPAD clinical trial, in which patients with peripheral arterial disease received treatment with paclitaxel-coated (drug-coated balloons or...
PAVE trial finds “no evidence of a benefit” from paclitaxel-coated balloons...
There is no evidence of a benefit from additional paclitaxel-coated balloon use compared to standard balloon angioplasty alone in the context of preserving arteriovenous...
Paclitaxel situation “crystallising” thanks to new data and teamwork, VIVA audience...
This year's Vascular Interventional Advances annual meeting (VIVA 2020; 6–8 November, online) opened with a session on controversial hot topics and advanced multidisciplinary approaches...
ILLUMENATE four-year pooled analysis has “brought increased clarity” to the paclitaxel...
Sean Lyden (Cleveland, USA), current VIVA Board president, speaks to Vascular News about his two years at the helm, which began with the “controversy on...
Vascular surgeons encouraged to consult talking points document on paclitaxel devices
Vascular surgeons are being encouraged to take consideration of a set of talking points about the risks and benefits of paclitaxel-equipped devices—which has developed...
Calls in Germany for reimbursement as analysis shows drug-eluting technology is...
Stefan Müller-Hülsbeck (Ev Luth Diakonissenanstalt zu Flensburg, Flensburg, Germany) presented attendees of the online meeting of the Cardiovascular and Interventional Radiological Society of Europe...
“No signal of increased risk of long-term mortality” with paclitaxel-coated Luminor...
An ad hoc, two-year analysis of the EffPac study found no signal of increased risk of long-term mortality, nor any adverse events, within two...
New prediction model for target lesion revascularisation grants “new level of...
“Please feel free to utilise this in your practice,” Michael Dake (University of Arizona Health Sciences, Tucson, USA) urged on the first day of...
Six-month IN.PACT AV Access results published, show superiority of DCB angioplasty
Just-published six-month results of the IN.PACT AV Access study show that drug-coated balloon (DCB) angioplasty is superior to standard angioplasty for the treatment of...
Intermittent claudication: EffPac trial confirms benefit and safety of paclitaxel-coated balloon...
In the EffPac trial, designed to compare a drug-coated and an uncoated balloon in the treatment of vascular occlusion in the femoropopliteal region, the...
Real-world analysis demonstrates lower long-term mortality after DCB angioplasty of femoropopliteal...
In a real-world retrospective analysis, recently published in JACC: Cardiovascular Interventions, the long-term mortality rate was lower after drug-coated balloon (DCB) angioplasty than after plain...
MHRA: Warning to be added to paclitaxel device IFUs in Europe
In a new field safety notice, the UK Medicines and Healthcare products Regulatory Agency (MHRA) states that a warning and clinical summary section will...
CX 2020 LIVE pioneers virtual vascular conference format in COVID-19 era
CX 2020 LIVE came to life online—despite COVID-19—using state-of-the art broadcast technology to bring together more than 1,000 vascular specialists, live, from 95 countries across...
VIVA Physicians publishes analysis evaluating mortality and paclitaxel-coated devices
An individual patient-level data (IPD) analysis of the safety of paclitaxel-containing devices (PTXD), conducted by VIVA Physicians, identified an absolute 4.6% increased mortality risk...
Real-world data with long-term follow-up confirm no increased mortality signal with...
Data from an unselected, real-world cohort of 64,000 claimants of the German BARMER Health Insurance reveals no signal of increased long-term mortality when paclitaxel-based...
Sirolimus on the rise but paclitaxel “will probably come back again”
At this year’s Leipzig Interventional Course (LINC 2020; 28–31 January, Leipzig, Germany), William Gray (Main Line Health, Philadelphia, USA) considered the future of drug-coated...
Impressions from LINC 2020: Strong clinical and economic case for widespread...
NOTE: This video is ONLY available to watch in selected countries and geographies
Andrew Holden (Auckland, New Zealand), Alexandros Mallios (Paris, France), Robert Lookstein (New...
Paclitaxel mortality signal inconsistent across different geographies
While drug-coated balloons (DCBs) have been “consistently efficacious” across various studies, the mortality signal has not, Peter Schneider (University of California San Francisco, San...
New meta-analysis finds “no observed difference” in mortality between paclitaxel and...
February 2020 brings another paclitaxel device meta-analysis of randomised controlled trials in chronic limb-threatening ischaemia (CLTI) patients. Krystal Dinh (Westmead Hospital, Sydney, Australia) et...
LINC 2020: Geographical “inconsistencies” cast doubt on paclitaxel mortality signal
Peter Schneider (San Francisco, USA) talks to BLearning Peripheral at LINC 2020 (Leipzig Interventional Course; 28–31 January 2020, Leipzig, Germany) about the relevance of dose relationship and geographical...
LINC 2020: Real-world evidence shows no increased mortality signal with paclitaxel-coated...
Dierk Scheinert (Leipzig, Germany) moderates a panel with Eva Freisinger (Münster, Germany) and Thomas Zeller (Bad Krozingen, Germany) to discuss the developments over the...
LINC 2020: High- and low-dose paclitaxel-coated balloons exhibit comparable results at...
At the 2020 Leipzig Interventional Course (28–31 January, Leipzig, Germany), Sabine Steiner (University of Leipzig, Leipzig, Germany) presented one-year results of the COMPARE-RTC of...
VEITH 2019: “Light at the end of the tunnel” following paclitaxel...
Sean Lyden (Cleveland, USA) talks to VEITHtv at the VEITHsymposium 2019 (19–23 November, New York, USA) about the “tumultuous” year for the industry following the publication of the...
Hotly-contested meta-analysis suggests a higher risk of death or amputation at...
A new meta-analysis, just published in the Journal of Vascular and Interventional Radiology (JVIR), suggests significantly worse amputation-free survival at one year with the...
Vascular surgeons and cardiologists respond to new meta-analysis of PCBs in...
Eminent vascular surgeons and interventional cardiologists including Kim Hodgson (Springfield, USA), Ramon Varcoe (Sydney, Australia) and Gary Ansel (Columbus, USA) give their thoughts on...
IR experts respond to new meta-analysis of paclitaxel-coated balloons in infrapopliteal...
A range of interventional radiology experts including Michael Dake (Tucson, USA), John Kaufman (Portland, USA), Jim Reekers (Amsterdam, The Netherlands) and Gunnar Tepe (Rosenheim,...
German claims data report higher long-term and amputation-free survival in CLTI...
There is no sign of increased all-cause mortality following the use of paclitaxel-coated devices for the treatment of symptomatic peripheral arterial occlusive disease, a...
Reaction to the Katsanos et al meta-analysis
Internal BIBA MedTech data show that perceptions on paclitaxel devices have shifted considerably in the twelve months that have elapsed since Konstantinos Katsanos (Patras,...
CIRSE 2019: Hear the latest from clinical trials comparing paclitaxel technologies...
NOTE: ONLY intended for healthcare professionals outside of the USA and France.
Zoom in on a tête-à-tete between Marianne Brodmann (Graz, Austria) and Giovanni Torsello...
CIRSE 2019: IN.PACT AV access trial meets primary safety and effectiveness...
Medtronic has announced the first-ever results from the IN.PACT AV access clinical study comparing the investigational IN.PACT AV drug-coated balloon (DCB) to percutaneous transluminal...
BASIL-3 resumes randomisation
The BASIL-3 study, which was halted in response to findings reported in the Journal of the American Heart Association (JAHA) that paclitaxel-coated and –eluting...
Medtronic statement regarding updated FDA letter to healthcare providers on paclitaxel...
Medtronic has issued the following statement regarding the US Food and Drug Administration’s (FDA) updated letter to healthcare providers for paclitaxel-devices in patients with...
FDA: Paclitaxel device clinical trials “may continue”; Agency is working to...
Clinical studies of paclitaxel-coated balloons and paclitaxel-eluting stents “may continue and should collected long-term safety (including mortality) and effectiveness data”, says the US Food...
FDA notifies BD that Lutonix paclitaxel balloon PMA application for below...
The US Food and Drug Administration (FDA) have denied BD premarket approval (PMA) for the company’s Lutonix drug-coated balloon (DCB) in the treatment of...
FDA notifies BD that Lutonix paclitaxel balloon PMA request for below...
The US Food and Drug Administration (FDA) have denied BD premarket approval (PMA) for the company’s Lutonix drug-coated balloon (DCB) in the treatment of...
Interim results of a multicentre analysis support paclitaxel-coated balloon angioplasty
The interim results of a multicentre, European retrospective analysis investigating the use of drug-coated balloons (DCBs) in symptomatic central venous stenosis (CVS) suggest that...
Webinar: Safety of paclitaxel devices—What does the evidence say?
Jos van den Berg (Lugano, Switzerland), Antonio Micari (Bergamo, Italy) and Marianne Brodmann (Graz, Austria) sit down after a specially convened US FDA panel...
C3 2019: Highlights from the 15th annual meeting
Rajesh Dave (Camp Hill, USA) talks to iWounds News about his highlights from the 15th annual C3 conference, which included a special session on...
Medtronic and BD respond to FDA circulatory system devices panel
Both Medtronic and BD have released statements in response to the FDA circulatory system devices panel held last week (19–20 June, Washington, DC, USA)....
SVS VQI data being “fortified” to shed light on long-term paclitaxel...
Kim Hodgson (Springfield, USA), president of the Society for Vascular Surgery (SVS) for 2019–20 spoke to Vascular News at the organisation’s Vascular Annual Meeting...
US FDA panel reviews paclitaxel device data: No recommendations issued, as...
The US Food and Drug Administration (FDA) convened this week for a General Issues Panel Meeting on the late mortality safety signal associated with...
Society for Vascular Surgery’s VQI adds new analysis to paclitaxel device...
The Society for Vascular Surgery (SVS) Vascular Quality Initiative (VQI), with its large multicentre registries has produced several datasets for analysis of vascular patient...
Holden et al find no causal link between paclitaxel dose and...
The methodology underpinning the conclusion by Konstantinos Katsanos (Patras, Greece) et al that there is a positive dose-response relationship between paclitaxel and mortality is...
Highlights from the CX 2019 session, Paclitaxel: The last word
Watch the video roundup from the CX 2019 Highlight Session, Paclitaxel: The last word.
Filmed onsite at the Charing Cross Symposium (15–18 April 2019, London,...
MHRA issues medical device alert for paclitaxel use in the UK
Do not use paclitaxel drug-coated balloons (DCBs) or drug-eluting stents (DESs) in the routine treatment of patients with intermittent claudication until further notice, a...
MHRA limits routine clinical use of paclitaxel devices following expert advisory...
The UK Medicines and Healthcare products Regulatory Agency (MHRA) acted to limit the future use of paclitaxel-eluting stents and paclitaxel-coated balloons in routine clinical...
SWEDEPAD trials set to resume enrolment
Investigators of SWEDEPAD have announced the conclusion of their safety committee analysis, which recommends the halted trials resume enrolment.
This decision to consider resuming enrolment...
The impact of paclitaxel concerns on vascular access
At the 2019 Charing Cross Symposium (CX; 15–18 April, London, UK), there was a section of the programme on drug-coated balloon trial updates which...
Cook Medical releases patient-level data from Zilver PTX paclitaxel-eluting stent study
Cook Medical has released de-identifiable patient-level data from a clinical trial of its Zilver PTX peripheral paclitaxel-eluting stent.
The move comes a month after the...
Hear the latest thoughts on paclitaxel from global experts: Their current...
Following the latest information presented at the Charing Cross (CX) International Symposium related to the meta-analysis of paclitaxel-coated devices (Katsanos et al), hear from a...
CX 2019: Evidence supports safety of paclitaxel-coated devices
At the Charing Cross International Symposium, Gary Ansel (Columbus, Ohio) moderates a global panel that includes Thomas Albrecht (Berlin, Germany), Peter Schneider (San Francisco,...
CX 2019: Cook leads the way to data transparency with release...
There were calls for greater transparency and sharing of the available randomised controlled trial data of paclitaxel-coated devices at the CX 2019 Highlight Session, Paclitaxel:...
While CX audience deems paclitaxel not dangerous, vascular pathologist says establishing...
Delegates voted overwhelmingly against the notion that there was a demonstrable danger in any organ of the body attributed to circulating paclitaxel. These polling...
Surmodics provides update regarding TRANSCEND clinical trial
Surmodics has announced the company has now resumed patient enrolment into its TRANSCEND clinical trial, and is nearly 75% of the way to its...
CIRSE updates position on paclitaxel use in peripheral arteries
The Cardiovascular and Interventional Radiological Society of Europe (CIRSE) has released a statement on the use of paclitaxel-coated balloons and stents in the treatment...
Update from the SVS paclitaxel safety task force
In response to the updated letter to healthcare providers from the US Food and Drug Administration (FDA), the Society for Vascular Surgery (SVS) released...
UK MHRA forms Expert Advisory Group to review paclitaxel devices while...
The Medicines and Healthcare products Regulatory Agency (MHRA) in the UK and the Food and Drug Administration (FDA) in the USA have both recently...
Pooled analysis of four RCTs finds no increased mortality with paclitaxel-coated...
A new paper looking specifically at mortality has found no difference in deaths between paclitaxel-coated balloons and uncoated balloons when used in the femoropopliteal...
Sirolimus has “a much wider” safety window and shows fewer local...
"Paclitaxel kills the cells in the artery wall; sirolimus simply stuns them," says Peter Gaines (Sheffield, UK). Gaines commented at the 2019 Vascular Leaders...
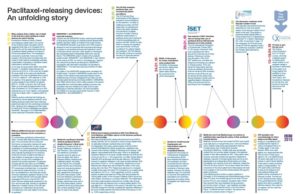
Paclitaxel-releasing devices: An unfolding story
Paclitaxel Timeline:
Meta-analysis finds a higher risk of death in the long term when paclitaxel-coated devices are used in the leg
Without additional long-term...
VLF 2019: White paper and individual patient data analysis are planned...
Krishna Rocha-Singh (Springfield, USA) tells Vascular News that plans after VLF 2019 include publishing a white paper and expeditiously pooling individual patient-level data from...
Revised safety analysis of Medtronic’s IN.PACT Admiral DCB published in JACC
Medtronic has issued the following statement regarding revised clinical study data:
On 15 February, 2019, Medtronic issued a statement regarding a programming error in the...
Vascular Leaders Forum discussions seek consensus on paclitaxel
The Vascular Leaders Forum (VLF, 1–2 March, Washington DC, USA), hosted by the non-profit organisation VIVA Physicians, is a special consortium which was called...
“No evidence of increased long-term mortality” with paclitaxel-eluting stent in new...
“Drug-eluting stents (DES) are important additions to the armamentarium of devices used for peripheral artery revascularization, associated with decreased rates of restenosis and target...
Medtronic revises IN.PACT post-market study data due to programming error, but...
Medtronic has issued the following statement regarding revised clinical study data:
Recently, Medtronic became aware of a programming error in the clinical data reporting isolated...
New JAMA analysis with short follow-up finds “an association of survival”...
A recent US-wide, multicentre analysis has found no evidence of increased all-cause mortality associated with the use of paclitaxel-coated devices compared with non-drug-coated devices...
Passeo-18 Lux DCB remains safe and effective at two years with...
Two-year data on the Passeo-18 Lux drug-coated balloon (DCB; Biotronik) continue to validate its safety and effectiveness in intra-inguinal arteries. Gunnar Tepe from the...
Patient-level analysis shows no correlation between paclitaxel and mortality—reactions from LINC
Renowned experts expressed confidence in the continued use of paclitaxel-coated devices after independent patient-level data presented at LINC 2019 did not show correlation between...
New data release at LINC 2019 reinforces safety profile of low-dose...
Sean Lyden (Cleveland, USA) and Fabrizio Fanelli (Rome, Italy) discuss the safety and future of drug-coated balloons (DCB) in light of the latest pooled...
ISET Paclitaxel Town Hall: Vast majority of attendees will not change...
The discussion around the use of paclitaxel-coated and eluting devices continues at the 31st International Symposium on Endovascular Therapy (ISET; 27-30 January 2019, Hollywood, USA). Organisers...
SCAI supports continued use of paclitaxel-coated balloons
Experts from the Society for Cardiovascular Angiography and Interventions (SCAI) have reviewed the recent meta-analysis by Katsanos and colleagues and have concluded that the...
Latest data from Philips reinforces the safety profile of Stellarex low-dose...
A new pooled analysis of patient-level data from Philips demonstrates the “strong safety profile” of the company's Stellarex drug-coated balloon (DCB) in above-the-knee studies,...
Patient-level survival analysis demonstrates no link between paclitaxel dose and mortality...
New data on the IN.PACT Admiral drug-coated balloon (DCB; Medtronic) in patients with peripheral arterial disease (PAD) in the superficial femoral (SFA) and popliteal...
Data presented at LINC confirm the safety and effectiveness of Zilver...
Findings presented at the Leipzig Interventional Course (LINC; 22–25 January 2019, Leipzig, Germany) contradict the results of a meta-analysis published in the Journal of...
IN.PACT independent patient-level meta-analysis shows no correlation between paclitaxel exposure and...
A renowned panel, moderated by Dierk Scheinert (Leipzig, Germany), comprising Peter Schneider (Honolulu, USA), John Laird (St Helena, USA) and Thomas Zeller (Bad Krozingen,...
US FDA evaluating paclitaxel data, recommend patient surveillance
The impact of the 2018 meta-analysis of randomised paclitaxel-device trials, published in the Journal of the American Heart Association by Konstantinos Katsanos et al,...
Medtronic and Boston Scientific stand by paclitaxel devices despite Katsanos’ critical...
Speaking to investors at the JP Morgan healthcare conference in San Francisco, executives from Medtronic and Boston Scientific said their data do not show...
SWEDEPAD 1 and SWEDEPAD 2 trials halt inclusion after Katsanos et...
Vascular News has learned that inclusion into the SWEDEPAD studies, that are examining benefits of drug-eluting technology for peripheral arterial disease patients, has been...
Meta-analysis finds a higher risk of death in the long term...
New data, just published in the Journal of the American Heart Association (JAHA), suggest that there is an increased risk of death at two...
Lutonix BTK IDE: First Level 1 evidence of positive safety and...
Patrick Geraghty (St Louis, USA) presented new datapoints from the six-month outcomes of the Lutonix BTK IDE trial at the 2018 VEITHsymposium (13–17 November,...
Lutonix 014 DCB gathers first randomised evidence of positive safety and...
Six-month outcomes of the Lutonix BTK IDE trial, a multicentre randomised controlled trial using the Lutonix 014 drug-coated balloon (DCB; BD), has demonstrated that...
Paclitaxel-eluting stent loses BATTLE against bare metal stent
The BATTLE trial comparing a drug-eluting stent (Zilver PTX, Cook Medical) vs. a bare metal stent (Misago, Terumo) for the treatment of intermediate femoropopliteal...
First data from head-to-head comparison of Ranger and IN.PACT drug-coated balloons...
Early data from the first randomised controlled trial to compare two drug-coated balloons (DCBs) suggest that the primary patency obtained with the Ranger DCB...
“Promising” 12-month outcomes achieved with the Chocolate Touch drug-coated balloon
The final results of the ENDURE study, investigating the efficacy of the Chocolate Touch drug-coated balloon (QT Vascular), show “promising evidence of the drug...