On the 3 February, the National Institute for Health and Care Excellence (NICE) Medical Technologies Advisory Committee approved the use of Leukomed Sorbact (Essity) for the prevention of surgical site infections.
The dressing works by binding bacteria to the dressing itself, which is then removed at dressing change. Approval was given for two indications, post-caesarean section or vascular surgery, and for closed wounds where low-to-moderate exudate is expected. Cost modelling suggests a saving of £107 per person after caesarean section and £18 per person after vascular surgery.
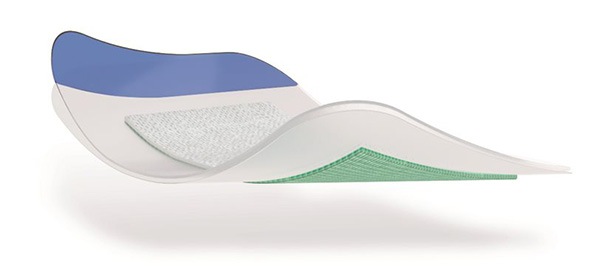
The NICE guidance describes Leukomed Sorbact as a sterile, single-use, bacteria-binding, adhesive-bordered wound dressing. In part, it explains: “The dressing comprises an absorbent non-woven wound contact pad and an outer transparent adhesive polyurethane film. The pad is made of a white viscose polypropylene and polyester mesh that is coated with the proprietary compound dialkylcarbamoyl chloride (DACC). DACC is hydrophobic, meaning that it does not mix with water and tends to bind to itself or other hydrophobic materials if water is present. In a moist wound, DACC binds to hydrophobic bacteria and fungi that cause surgical site infections. These bound microorganisms are then removed from the wound site when the dressing is changed. Binding to DACC does not cause bacteria to be lysed (broken open), which avoids causing inflammation at the wound site. The polyurethane film is designed to maintain a moist environment and protect the wound from external contamination. The dressing is available in various sizes.”










