
“Diabetic foot ulcers are very difficult to treat, and the approach should be multi-factorial,” Andrew Applewhite tells iWounds News. “If you do not offload a diabetic foot ulcer, if you do not treat any infection present, if you do not ensure there is adequate circulation, then no matter what you do, you will have a very difficult time healing those wounds. In a select group of patients, hyperbaric oxygen therapy can be used as an adjunct treatment to treat these hard-to-heal wounds.”
The five-year mortality rate after a major amputation is approximately 50%; Applewhite refers to it as a “devastating, life-changing event”. By preventing these amputations, physicians treating patients with Wagner Grade III or worse diabetic foot ulcers significantly improve the quality of life of these patients. Hyperbaric oxygen therapy is one healing modality that has earned its place in the treatment paradigm for appropriately-selected patients, Applewhite enthuses. He details the data so far published on the therapy, and outlines the ethical and logistical difficulties with obtaining more—though he adds that while further robust evidence would be “nice”, it is also “unnecessary”, as the evidence so far published in the literature, in combination with his experience of treating patients with hyperbaric oxygen therapy for nearly two decades, confirms to him the effectiveness of the intervention.
He says of watching his patients slated for major amputation walk away on two good feet: “That makes an impact”.
What is hyperbaric oxygen therapy and how does it work?
Let’s break it down: “hyper” means elevated; “baric” means pressure; “oxygen” is oxygen. Typically, at sea level, you are breathing 21% oxygen at one atmosphere of pressure. What we do in hyperbaric oxygen therapy is we place the patient in a pressure chamber, increase the pressure to about two-and-a-half times what normal atmospheric pressure is—that is equivalent to roughly 45 feet of sea water—and then give 100% oxygen. Essentially, the patient is breathing 100% oxygen at elevated atmospheric pressures.
This forces oxygen to be carried not only on the red blood cells, which can get saturated with oxygen, but it dissolves the oxygen molecules directly into the plasma, so we do not have to rely on the red blood cells to carry oxygen throughout the circulatory system. That means that the patient’s blood is super-saturated with oxygen, carrying 20 times more than the body would normally carry. In addition, red blood cells are fairly bulky cells, and an oxygen molecule is very small, so an oxygen molecule can get to places that a red blood cell cannot—for example, in an atherosclerotic passage, where a blockage might prevent a red blood cell from entering. Further to getting hyper-oxygenated tissue right away, [hyperbaric oxygen therapy also leads to a steep oxygen gradient between the blood plasma and surrounding tissues]. That gradient between the high oxygen and the low oxygen can also regrow a capillary bed in a process called angiogenesis. This can give long-lasting effects; over the course of six to eight weeks, you can actually regrow that capillary bed that has been destroyed by certain disease processes, such as diabetes.
Hyperbaric oxygen therapy decreases inflammation, bolsters the body’s immune system, and improves the leukocyte-killing capacity, so the white blood cells work better. It does a number of different things, and addresses a lot of the different problems that you see in diabetic foot ulcers.
Who is the ideal patient for this therapy, and when would you use hyperbaric oxygen therapy in the treatment algorithm?
Current recommendations based on the studies and data that are out there advise using hyperbaric oxygen therapy as an adjunctive therapy; it is not the only thing to do for a patient. It has got to be part of a wider treatment plan. It has got to be used in conjunction with treating infection, off-loading—I cannot emphasise enough how important off-loading is—and revascularisation. Say the patient has a blockage of the superficial femoral artery; no matter what you do, if the blood is not getting past that blockage, then you are not going to save the foot. So you have to revascularise as much as possible.
The typical, ideal patient, then, would be a patient with a Wagner Grade III diabetic foot ulcer (a deep wound that involves the joint or bone), who has still failed traditional wound care therapy after approximately 30 days, has been maximally revascularised, has been off-loaded, and whose infection has been treated. If the wound is still not healing, they would be a good candidate for hyperbaric oxygen therapy. It should not be considered a Hail Mary; it should be considered a part of the treatment regimen in specific types of wounds. In conjunction, you have to make sure that their sugars are being controlled and their protein levels are adequate; it is a multi-factorial process, you cannot look at it in a silo, treatment has to be all-encompassing.
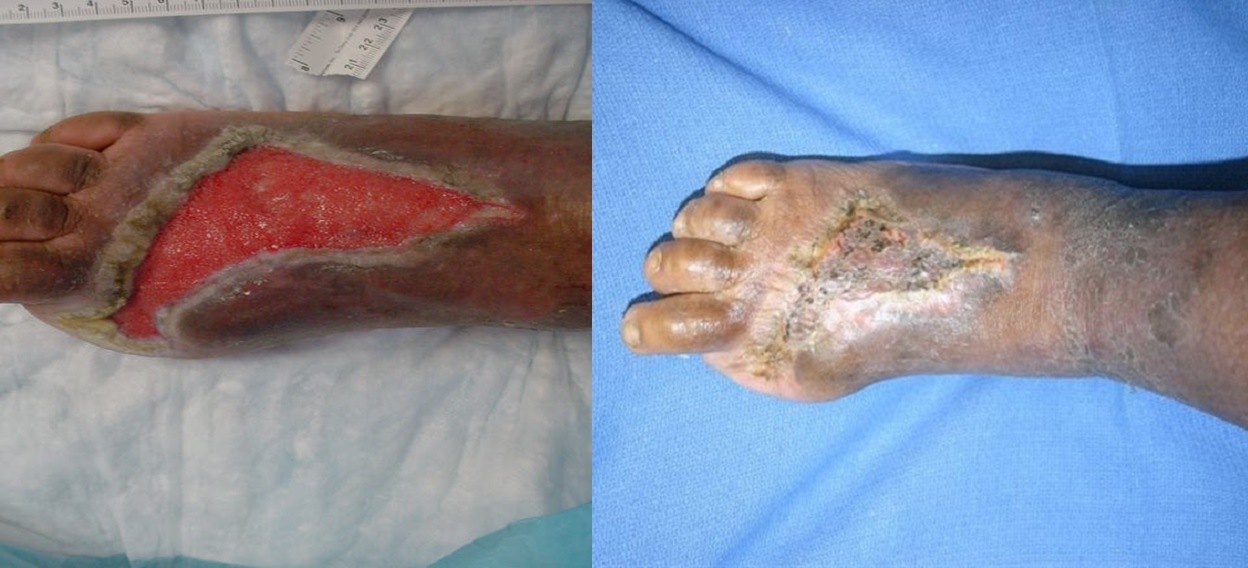
Some people are sceptical of this therapy; why do you think that is?
I think the reasons [for scepticism] are multi-factorial. I think you can get blinders on and look at it [hyperbaric oxygen therapy] in and of itself. I think there are a lot of people out there who do not use it as part of the process, they use it as the only process. Here in the USA, there are a lot of people who are treating diabetic foot ulcers, but are not really addressing a lot of the problems, such as off-loading, such as making sure the infection is being treated, and glycaemic control. Also, it is expensive, so that is an element.
There are enough data that insurance companies and our Medicare programme cover it, but the coverage is very specific: you have to have evidence that it is a Wagner Grade III wound or higher, it has been present for over 30 days, has been treated and has not improved, you are offloading, you have revascularised, and the infection is being treated. Hyperbaric oxygen therapy is part of a treatment plan, it is not the one and only [therapy option]. But people see it as a very expensive part of that plan. However, while the up-front costs are expensive, compared to the cost of an amputation over the course of a patient’s lifetime, it has actually been shown to decrease costs. It can be cost-effective if used appropriately.
If it is used inappropriately, it is not going to be effective! It could be superfluous (if you use it on a wound that is going to get better anyway, such as Wagner Grade I and II wounds, then it is not going to help), or if you use it on a wound that is already two steps of the way to an amputation, then you may be too late, so you need to find that sweet spot.
Could you describe the current data for hyperbaric oxygen therapy?
There has been a lot of evidence. Back in 2012/ 2013, there were two systematic reviews into interventions with diabetic foot ulcers. They looked at all of the data that was out there, and both studies concluded that hyperbaric oxygen therapy resulted in higher healing rates and lower risks of major amputations (above-the-knee and below-the-knee amputations), but they did note that minor amputations, such as toes, remained about the same. So if the toe is dead, it is not going to save it with hyperbaric oxygen therapy.
Again, the preponderance of the data is positive, leading to Medicare and insurance company coverage in very specific circumstances. Patient selection is key. However, there were a couple of dissenting articles written in 2016 and 2017.
One was by Ludwik Fedorko (Toronto General Hospital, University Health Network, Toronto, Canada) et al. They looked at the evidence and concluded that hyperbaric oxygen therapy does not offer any advantage. [Published in Diabetes Care, this prospective, double-blind, randomised controlled trial concluded that hyperbaric oxygen therapy does not reduce indications for amputation in patients with diabetes with non-healing ulcers of the lower limb.] The problem with that study is they include Wagner Grade II diabetic ulcers, which would heal on their own, and they did not standardise wound care—they did not off-load everybody, it was not a really robust study, in that there was a lot of issues with it.
Then Katrien T B Santema (Academic Medical Center, Amsterdam, The Netherlands) and colleagues looked at the same thing, essentially, and they came to similar conclusions [also published in Diabetes Care], although their numbers were a little bit better for the hyperbaric groups. Again, pretty low numbers. They also included Wagner Grade II diabetic foot ulcers, so it is hard to look at these if you are not choosing the right patients. In addition, Santema et al did not standardise care; they did not say they off-loaded anything, they did not say how many real treatments they did, and only 65% of patients in the Santema trial received a full course of 30 treatments. So there are some issues with both of those studies. If you do not define the types of patients that are going to benefit from a therapy, and treat those patients with it, then it is very difficult to really look at the effectiveness of what you are studying.
The overall reviews, going back to the late ‘70s and early 80s, of hyperbaric oxygen therapy when used in those specific patient criteria wounds, show a very good response to the treatment in these diabetic foot ulcers.
What future work would you like to see on hyperbaric oxygen therapy?
I would love larger tests, with a very defined treatment protocol with standardised wound care and randomising the patients into getting hyperbaric oxygen therapy or not getting hyperbaric oxygen therapy. The problem is that I believe we have enough data to say that it does benefit certain patients, and so not treating a patient when hyperbaric oxygen therapy would be appropriate for them I think does them a disservice and could lead to potentially devastating results. I would feel uncomfortable not treating somebody that I feel would benefit from hyperbaric oxygen therapy.
One way to get around this quandary is that there are places where it is not available. If you could standardise the wound care the patients are getting, and define their vascular status and their glycaemic control, [you could compare these cohorts]. It is difficult to standardise these patients, though, as they are all different. It would almost take an insurmountable amount of time, and effort, and money to really get another big robust trial. The problem is, oxygen is cheap, and nobody wants to pay for these studies—it is not like a drug, which you could make a lot of money off.
The other part of the problem is trying to double-blind patients and physicians—it is very difficult to pressurise somebody without them knowing what treatment they are getting, and the treating physician not knowing. It can be done: you can adjust the gas mixture on the sham treatments to semi-equilibrate breathing surface oxygen, but it is very difficult to do, and there are not a lot of places that are equipped to do that. I think there are a lot of other areas of research into hyperbaric oxygen therapy where the resources would be better invested.
There are clearly obstacles to doing a larger study, therefore, including some ethical qualms about not giving appropriate patients hyperbaric oxygen therapy, so it is my opinion that while it would be nice to have these larger trials, ultimately they are not going to happen, and that is OK because we do have enough data.
Anecdotally, I have been treating foot ulcers with hyperbaric oxygen therapy for almost 20 years, and I have seen it prevent amputations. The patient who comes to you and they have already been told they need an amputation, and they walk out of your office on two good feet—that makes an impact.

Are you convinced by the data for hyperbaric oxygen therapy?
I am. I look at the data, and I treat patients appropriately, and I see the outcomes. In my centre, about 70–85% of the diabetic foot ulcers we see are devastating to these patients; these are patients who are told they need amputations, and unfortunately you cannot save them all, because some you may get to too late. But my numbers [for success with hyperbaric oxygen therapy] mirror the numbers of the data that are out there, and that is from a career that has spanned 18 years in this arena.
Andrew Applewhite is a wound care specialist at Baylor University Medical Center, Dallas, USA. He has no disclosures relevant to this article.












