Sky Medical Technology has announced that the geko device was presented as part of the didactic program, and at a corporate-sponsored symposium, at the Orthopaedic Summit 2019: Evolving Techniques (OSET 2019) annual meeting (December 11–14, Las Vegas, USA).
Jason Snibbe, a fellow from the Kerlan-Jobe institute, University of Southern California (USC) team surgeon, and an orthopaedic surgeon practicing in Los Angeles, USA, presented a real-world total knee replacement (TKR) patient case study to an audience of over 900 orthopaedic surgeons and healthcare professionals. The case focused on the patient’s range of motion and mobility issues following total knee arthroplasty, where oedema was determined as the culprit.
Snibbe presented the clinical rationale and his use of the FDA-cleared geko device to prevent the build-up of oedema on the lower extremity orthopaedic procedures he performs. Snibbe later presented in a corporate-sponsored symposium further information, titled “Postoperative oedema in orthopaedics-clinical implications and an innovative solution”, along with Brian Loyd (University of Utah, Salt Lake City, USA).
Postoperative oedema occurs in over 90% of total hip and knee replacement patients and is one of the main postoperative challenges where there is no dependable solution. Attendees heard from faculty thought-leaders on the economic burden oedema creates, as well as detail on this disruptive technology that, according to a statement, provides a long needed solution for the orthopaedic community.
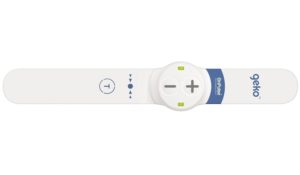
The geko device is the size of a wristwatch and worn at the knee. Through an innovative mechanism of bioelectronic neuromuscular electrostimulation, the daily disposable device gently stimulates the common peroneal nerve activating the calf and foot muscle pumps, resulting in increased blood flow in the deep veins of the calf for the prevention and treatment of pre and postoperative edema and the prevention of venous thromboembolism (VTE), in both surgical and medical patients at risk of VTE.
Evidence presented also demonstrated that swelling complications are experienced beyond 50 days, continuing in many patients for over three months, as well as trial data demonstrating the geko device’s prevention of postoperative oedema build-up in total hip replacement patients.










