The 2019 Prevention and Treatment of Pressure Ulcers/Injuries: Clinical Practice Guideline, published on 15 November 2019, has introduced new evidence-based recommendations for using a “subepidermal moisture (SEM)/oedema measurement device as an adjunct to routine clinical assessment.”
“The days of subjective skin assessments of this #1 reported patient harm being the only tool available to nurses in their diagnosis of PU/PIs are over. We are very pleased that measurement of SEM is recommended by the new evidence-based clinical practice guidelines as an adjunct to the existing routine clinical assessment of the skin. Nurses will be significantly aided in their day-to-day work as a result of this,” said Martin Burns, CEO of BBI.
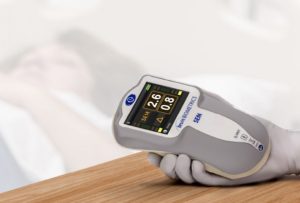
The SEM Scanner from BBI is the only US Food and Drug Administration (FDA) authorised and CE marked device for pressure ulcer risk assessment. “It is encouraging to see that the 2019 recommendations from global leading authorities and experts have recognised developments in the clinical understanding of pressure ulcer development including the role of SEM”, Burns added.
“Clinicians estimate that around 95% of PU/PI are preventable. Our singular objective is to reduce pressure ulcer incidence by helping clinicians make prevention real. Anatomy specific risk assessment using the SEM Scanner addresses chronic latency problems, pressure ulcer detection latency and interventional latency. Furthermore, the SEM Scanner is the only FDA Authorised and CE Marked device for this clinical purpose. Where we have seen our SEM Scanner in use, nurses have published significant decreases in hospital acquired pressure ulcers; a 93% reduction being the latest published number,” commented Burns.
The CPG are the global leading authority on the prevention and treatment of pressure ulcers and is comprised of three independent bodies: the National Pressure Injury Advisory Panel (NPIAP), European Pressure Ulcer Advisory Panel (EPUAP) and the Panel Pan Pacific Pressure Injury Alliance (PPPIA). In addition, these organisations work alongside other global experts, including those from Indonesia, China and Japan.
The SEM Scanner received European CE Mark approval in 2014 and is in full commercial use in the European Union, including the UK, Belgium and Spain. It received Health Canada clearance in 2016 and the US Food and Drug Administration (FDA) granted marketing authorisation in December 2018 for the SEM Scanner under its de novo review process for novel low- to moderate-risk devices that are not substantially equivalent to an already legally marketed device. More recently, the SEM Scanner was launched in Australia and New Zealand with TGA approval.












