Bard Peripheral Vascular has announced it has received CE marking to market the Covera Plus covered stent.
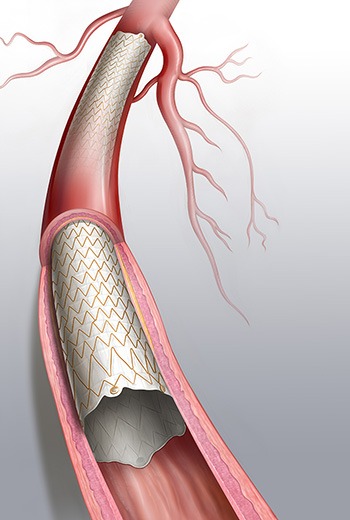
According to Bard, with the new Covera Plus vascular covered stent, the Bard Covera covered stent product family is expanding its treatment options to include stenoses in the upper extremity venous outflow of patients dialysing with an arteriovenous (AV) access graft or fistula and for the treatment of atherosclerotic lesions in iliac and femoral arteries with a reference vessel diameter of 4.5mm to 9mm.
In a press release, the company stated that the Covera Plus builds upon proven highly flexible stent architecture designed for tortuous anatomy and challenging fatigue conditions. The self-expanding nitinol stent is fully encapsulated within two layers of microporious ePTFE with carbon impregnation on the luminal surface designed to reduce early stage platelet adhesion.
A triaxial delivery system with two speed options, in combination with minimal implant shortening during deployment and highly radiopaque tantalum markers on the covered stent ends contribute to placement control and accuracy.













