This article forms part of an educational supplement sponsored by Shockwave Medical. Explore the full Shockwave L6 series here.

In this case report, Stefano Fazzini (University of Rome Tor Vergata, Rome, Italy) illustrates how the new Shockwave L6 offers an “ideal solution” for severely calcified iliac lesions as part of an optimised approach to imaging and functional assessment.
The external iliac artery (EIA) is a very tortuous segment with a high flexion point at the level of the inguinal ligament and usually presents with a smaller diameter compared with the proximal (common iliac artery [CIA]) and distal (common femoral artery [CFA]) segments. Therefore, considering a tapered diameter at the level of these locations, a stenting procedure can be complicated by anatomical mismatch of the stent where over/undersizing of the stent selection occurs. Occlusion of the collateral vessels, such as the internal iliac artery (IIA), is also a consideration when selecting a covered stent.
This is a case example of a 71-year-old man with lifestyle-limiting claudication of the left leg (Rutherford III, walking distance <50 meters). He was an active smoker affected by arterial hypertension, dyslipidaemia and obesity. The preoperative Duplex scan showed monophasic flow at the level of the left CFA with a significant stenosis at the origin of the left EIA. The ankle-brachial index (ABI) was 0.6; two further lesions (common femoral bifurcation and distal superficial femoral artery) were identified in the ipsilateral femoropopliteal segment.
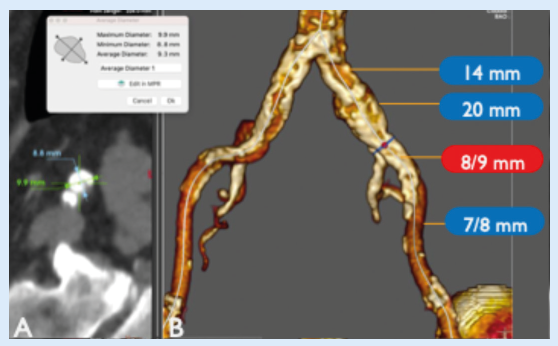
Computed tomography (CT) angiography (Figure 1) showed diffuse aortoiliac calcification on the left side, with a severe calcified eccentric stenosis at the origin of the EIA (with an ectatic CIA) and confirmed stenoses at the origins of the profunda and superficial femoral artery as moderate and severe, respectively.
In cases of severe claudication, our strategy is treating the main inflow, focusing the procedure to treat the external iliac lesion. Because of the calcific morphology of the plaque and the large vessel diameter, we believed that the patient could benefit from an endovascular treatment with the new Shockwave L6.
As per protocol in our institution, our plan was to stent the lesion only in case of complications (flow-limiting dissection or rupture) and/or suboptimal result of intravascular lithotripsy (IVL) treatment.
The reference vessel diameter (measured media-to-media) at the level of the EIA stenosis was between 8 and 8.5mm so we selected a 9mm Shockwave L6 catheter in order to have an ideal oversizing to facilitate optimised energy transfer to the vessel wall. Under local anaesthesia, a percutaneous left femoral access was performed positioning an 8F sheath. In this case, the 8F size has been used to ensure readiness in case of vessel rupture and subsequent use of a covered stent. The initial angiogram confirmed the presence of a tight and focal stenosis at the origin of the EIA and a chronically occluded IIA. The CIA diameter was between 14mm (proximally) and 20mm (distally); therefore, in case of stenting, a bare-metal stent was contraindicated for the risk of turbulence in this tapered segment (20mm>8mm) and two different covered stents or a single iliac limb of an aortic endograft would be required, due to the large proximal landing zone. The preoperative pressure gradient across the lesion was 41mmHg.
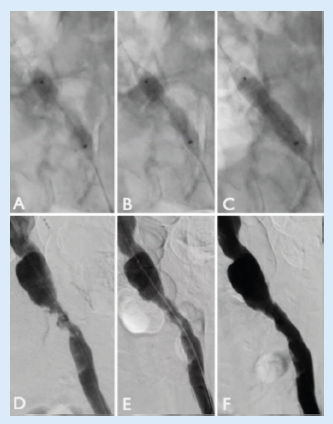
Pre-dilatation was performed with a 3.5x40mm plain angioplasty balloon and, thanks to the new 0.018” platform, the trackability of the Shockwave L6 made it easy to deliver into position. We proceeded to deliver all 10 cycles of therapy (five cycles at 2atm, five at 3atm; Figure 2). The completion angiogram (at least two different projections) showed an optimal result, without evidence of rupture or dissections, with a low residual stenosis <15%. Furthermore, the pressure gradient across the lesion after the treatment was <5mmHg and extravascular ultrasound (EVUS) at the level of the left CFA showed triphasic flow (Figure 3). The distal angiogram showed direct flow to the below-the-knee vessels and the left pedal pulse was present at the end of the procedure. We were satisfied with the post-IVL result and therefore we decided to avoid any stent and any further local or distal treatment.
The patient was discharged on the first postoperative day with an ABI of 0.9 (+50%) and single antiplatelet therapy, without any symptoms. Two months after the procedure a Duplex scan confirmed the success of the procedure with triphasic flow at the left CFA, left pedal pulse and complete resolution of the claudication.
This case is an example of three different methods to assess an ideal result, in term of imaging and functional assessment. In our daily practice this new approach (multiple angiograms + gradient pressure + EVUS) is becoming the standard to treat iliac lesions and allows us to leave nothing behind after optimal IVL treatment. This ‘no-stent option’ could be of benefit in different locations and for different reasons. Some of the benefits could be avoiding unnecessary kissing stents, maintaining the patency of collaterals, treating a tapered iliac segment, and avoiding stents that could be prone to fractures in the distal zone.
Shockwave L6 builds on the safety and efficacy of the heritage peripheral IVL catheter portfolio and provides an ideal solution for severely calcified lesions in the iliac arteries. This new concept of ‘leave nothing behind’ in the iliac territory is reshaping this kind of treatment and presents the opportunity to maintain future treatment options for our patients.
Stefano Fazzini is an associate professor of vascular surgery at the University of Rome Tor Vergata in Rome, Italy. He is a paid consultant of Shockwave Medical and his views expressed are not necessarily those of Shockwave Medical.
Case images