Joseph V Lombardi (Cooper University Hospital, Camden, USA) reported long-term outcomes favouring the continued safety and effectiveness of a composite device (proximal covered stent graft plus distal bare stent) deployed for the endovascular repair of acute, type B aortic dissection (TBAD) complicated by aortic rupture and/or malperfusion.
“STABLE II results place freedom from all-cause mortality at 80.3% at one year, and 68.9% at five years [with the use of the Zenith dissection endovascular system from Cook Medical]. Further, there were two procedure-related deaths, and the freedom from dissection-related mortality remained 97.1% at one year through five years,” he told delegates of the Society for Vascular Surgery’s Vascular Annual Meeting (SVS VAM 2021; 18–21 August, San Diego, USA and online).
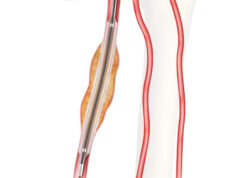
The Zenith endovascular system includes a proximal covered stent graft to cover the primary entry tear plus a distal bare metal stent, built on a nitinol Z-stent platform, for true lumen support. The bare metal component provides expansible support along the length of the dissected aorta without blocking critical branch vessels to provide a scaffolding to aid adjunctive interventions and facilitate favourable aortic remodelling of the entire dissected aorta.
Outlining the adverse events seen in the study, Lombardi commented that notable adverse events at one year were one instance of aortic rupture, one instance of conversion to open repair and two instances of stroke—there were no instances of paraplegia or paraparesis. “Freedom from secondary intervention was 88.2% in one year, and 70.7% at five years and secondary interventions were used to treat a number of presentations, including distal aortic growth. Aortic growth distal to the stent graft suggests the need for continuous monitoring,” he added.
Study details
STABLE II is a prospective, single-arm, multicentre study that enrolled 73 patients between August 2012 and January 2015 at institutions in the USA and Japan. The mean age of patients was 60.7 years with nearly 66% being male with acute TBAD complicated by malperfusion (78.1%), rupture (27.4%). Patients were treated with either a composite device (proximal covered stent-graft plus distal bare stent, 79.5%) or the proximal stent graft alone (no distal bare stent, 20.5%). Dissections were more extensive in patients receiving the composite device (408.9±121.3mm long, with 47.4% of dissections extending into the iliac arteries) compared with those who did not receive the distal bare stent (315.9±100.1mm long, with 6.7% of dissections extending into the iliac arteries). Five-year follow-up was available for 86.1% of eligible patients.
False lumen thrombosis and the composite device
The researchers also communicated that proximal dissection events were reported in seven patients, including four patients with retrograde progression of dissection and three patients with a new tear/type A dissection. Importantly, Lombardi called out the progressive false lumen thrombosis seen within the stent graft region and the dissected stent region. “Complete false lumen thrombosis rate increased (75% of patients at five-year vs. 51.4% post-procedure), with a higher complete thrombosis rate at five years in patients who received the composite device (81%) compared with the rate in patients who did not receive the distal bare stent (57.1%).
“There were no incidences of device fracture or infolding reported and these five-year data suggest a positive influence of composite device use on false lumen thrombosis,” he summarised.
Bare metal stent necessities
To a question posed on how the decision was made to deploy or not deploy the bare metal stent, Lombardi clarified: “We left that up to our investigators to determine the need for additional stenting using the bare metal stent. A lot of the dissections were very focal on the thoracic aorta and some thought that an additional dissection stent was not needed to support normal aorta distally. So, this was a primary reason why dissection stents were not deployed, but the fact that we did not have dissection stents in a small cohort of patients gave us a nice control group to look at afterwards.
When pressed on recommendations regarding when to use the dissection stent and tips on how far to place it, especially when the dissection was particularly distal, Lombardi explained: “The data is very supportive of the distal aortic remodelling using the dissection stent. And oftentimes, as you saw in the data, secondary interventions are not infrequent, and we will have to go back and reintervene for distal aortic growth to remedy ongoing expansion. The use of the dissection stent in that situation for me and for a lot of our colleagues has been very, very helpful in mitigating a continued false lumen flow. So, I am a believer in using it in all settings, as long as I’m putting it across dissected aorta.”