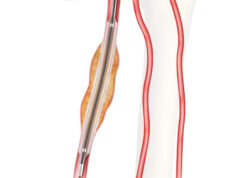
The Zenith fenestrated+ endovascular graft (ZFEN+) product (Cook Medical) has received breakthrough device designation from the US Food and Drug Administration (FDA). This designation is granted for devices that have the potential to provide more effective treatment or diagnosis for life-threatening or irreversibly debilitating diseases or conditions.
While the product is not commercially available yet, it will now receive priority review, and Cook will engage in “interactive and timely communication” with the FDA during the clinical trial and premarket review phases. The ZFEN+ is the first product from Cook Medical to receive a breakthrough device designation.
The product is intended for use in the endovascular treatment of patients with aortic aneurysms for whom the graft seal zone requires fenestrations and involves one or more of the major visceral vessels: celiac artery, superior mesenteric artery, and/or renal arteries.
The Zenith fenestrated+ endovascular graft includes up to five fenestrations to accommodate the visceral vessels. Cook say in a press release that this extra customisation allows the graft “to be tailored to the patient’s unique anatomy and maximise the seal zone to exclude the aneurysm”. Cook is seeking investigational device exemption (IDE) approval in the coming months with the intention of beginning a pivotal clinical study later this year.