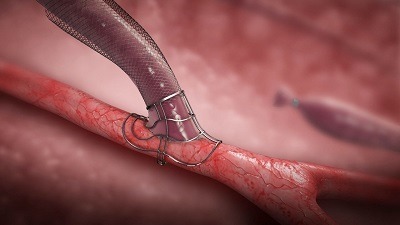
Laminate Medical Technologies has announced the enrolment of their first forearm fistula patients in their US pivotal trial of the VasQ device. VasQ is an implanted blood vessel external support intended to promote maturation and reduce the high primary failure rate of surgically created fistulae for haemodialysis. The forearm fistula is the gold standard for vascular access creation but also has the highest risk of primary failure. The inclusion of the forearm fistula to the US pivotal trial of VasQ represents a significant potential advancement for dialysis patients to help improve their overall care, the company states.
The first surgical VasQ implantation for a forearm fistula patient was performed by Samir Shah at the Brigham and Women’s Hospital in Boston, USA, who was quickly followed by Eric Peden of Houston Methodist and John Lucas of Greenwood Leflore Hospital. Lucas remarked, “There is a pressing worldwide need for technologies to improve fistula outcomes, and the early results with the VasQ device based on European data have been promising. I am excited about the potential of this device to assist the maturation of the fistulas that I create for my patients. VasQ was easy to place and the immediate outcomes were excellent.”
The VasQ US pivotal clinical trial is a prospective, multicentre, single-arm, open-label, 15-site study enrolling 129 male and female patients, 18–80 years old, who require creation of an arteriovenous fistula for haemodialysis. The original study protocol focused on upper arm fistulae, but now the study has expanded enrolment to include patients indicated to receive a forearm fistula. The primary effectiveness endpoint for this trial is the primary patency rate six months after creation of the arteriovenous fistula with VasQ implantation. Patients will be followed for a total of two years to additionally assess long-term durability.
“Following a very successful expansion in Europe to treat forearm fistula patients with VasQ, and excellent clinical outcomes that were presented in international conferences, the inclusion of forearm fistula patients in the pivotal study marks an important step toward VasQ market clearance in the USA and a promising milestone for patients with kidney failure”, said Tammy Gilon, CEO of Laminate Medical.
VasQ is an external support placed over the fistula at the creation site promoting a positive biomechanical response by optimising haemodynamics and minimising wall tension post-surgery, which leads to vein dilation instead of constriction. This reduces the risk of primary failure and allows the fistula to achieve sufficient blood flow during dialysis. Several studies in Europe and Israel have reported an overall low primary failure rate of VasQ fistulae relative to the standard of care. VasQ is a CE marked device already benefiting patients in Europe and Israel with impressive results. VasQ is currently under investigational use in the USA.












