This advertorial is sponsored by Bentley.

Bridging stents have been used in fenestrated and branched endovascular aneurysm repair (F/BEVAR) for many years. However, their use has always been off-label. In cooperation with Bentley, two investigator-initiated studies are set to create the necessary evidence to shift the use of balloon-expandable covered stents from off-label to on-label in complex aortic procedures.
Eric Verhoeven (General Hospital Nuremberg, Paracelsus Medical University, Nuremberg, Germany) is leading a study focused on bridging stents in FEVAR procedures, for which enrolment is almost complete. A similar study, headed by Martin Austermann (St Franziskus Hospital, Münster, Germany), is ongoing for BEVAR. Here, Verhoeven and Austermann speak to Vascular News about the importance of having on-label bridging stents, what makes the BeGraft stents (Bentley InnoMed GmbH) stand out, and how the FEVAR and BEVAR studies will pave the way for on-label bridging stent use and further innovation in the space.
Why is it so important to have a bridging stent available as on-label for F/BEVAR?
EV: We have used bridging stents in F/BEVAR cases for many years. In the past, however, companies were not interested in developing dedicated covered bridging stents, because they did not see the purpose. That changed when it became clear that both FEVAR and BEVAR enjoyed widespread use and were here to stay.
In addition, I think covered stents will be increasingly used in other diseases, like peripheral arterial disease (PAD), or in other small aneurysms and small vessels. So, while covered stents have a clear future in many different procedures, an on-label indication in FEVAR is needed. This is why it is great to see that a company like Bentley is supporting the FEVAR and BEVAR studies, which will result in an on-label indication for covered stents in complex aortic procedures.
MA: It is crucial that bridging stent grafts work in F/BEVAR procedures. If you exclude a complex aneurysm with involvement of the renal or the visceral arteries in fenestrated and branched repairs, it is vital that these bridging stents perform well. If they fail, the complete repair fails, and can lead to complications such as dialysis or an endoleak. So, we have to know how bridging stents perform in this indication, and a clinical study is the best environment in which to evaluate them and create the evidence.

Could you give a few details about the FEVAR study design and any key updates?
EV: We are almost at full enrolment, and so inclusion has been quite fast. The purpose is to include as many suitable patients/vessels as possible. About one-third to one-quarter of patients we regularly treat with FEVAR are not in the study, for reasons such as their target vessels being too small or having too sharp take-off angles. The endpoints are safety and effectiveness, so we are looking at patency and long-term outcomes. It is also important to note that the study is not linked to one dedicated fenestrated graft company; many patients were treated with Cook fenestrated grafts but we allow the use of other fenestrated stent grafts.
How do the BeGraft products compare to other covered stents on the market?
MA: In Münster, we have conducted some bench tests on the two grafts—the BeGraft and the BeGraft PLUS—over the past few years. Our results regarding the BeGraft PLUS, for branches, show that it is a very durable stent, with a double-layered stent and polytetrafluoroethylene (PTFE) design. We found that, after 100 million cycles in our machine, the stent performed far better than expected. Another important feature of the BeGraft PLUS is the kink resistance. You can bend the stent more than 90 degrees and it does not create a kink, it creates a curve.
The regular BeGraft stent, which is being assessed in the FEVAR study, has good flexibility, which is a big advantage due to the fact that the renal arteries move around a lot with every breathing movement.
Why do you think it has taken until now for these studies to be conducted?
MA: The market was very small for many years. Twenty years ago, a couple of centres started to use fenestrated and branched technologies, and, over the years, they showed that these procedures work and that patients survive. Physicians and patients started to observe that the use of fenestrated or branched devices resulted in better survival than open repair. In addition, it solved some other problems, like spinal cord ischaemia, for which we have now reduced the risk to around 2%. It was noted that bridging stents were crucial to these complex repairs. For many years, there was only one covered stent available, which was used off-label. However, as the procedure gained popularity, the market grew. Eventually, it became clear that a dedicated stent is necessary, and therefore I am happy that Bentley is supporting studies to learn more about bridging stents, which is the first step towards dedicated bridging stents for complex aortic procedures.
When can we expect the first outcomes of the FEVAR study and, further down the line, the on-label indication?
EV: I think once enrolment is complete, which will be very soon, we should be able to provide one-month results quite quickly. The rest is about durability. It is difficult to say when the on-label indication will be approved, because the process is thorough and can therefore take a long time.
Will the study change the FEVAR procedure?
EV: I think the study will change the FEVAR procedure, yes. For a long time now, there have been no on-label covered stents for complex aortic procedures, and so our only option has been to use what is available. When a covered stent gains an on-label indication, however, there will be a clear choice. The availability of a validated, on-label covered stent for fenestrated grafts will, I think, be a decisive advantage in the market, and also I believe other companies will move forward as a result.
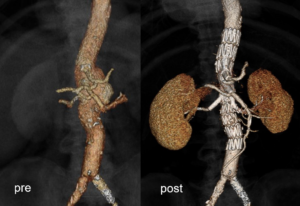
Where do things stand with the BEVAR study?
MA: We are still recruiting patients, but the first results seem very promising. We have faced some challenges, but this points to a big advantage of the study—that we are open to saying, ‘this stent works in this indication, but maybe this is a border, and we should respect it’. I think it is important to document all experiences—good and bad—in every study, and to have the next generation of devices in your head. Bentley has good stents available, but what makes our job so difficult is that no patient is the same, and therefore there is no ‘one-size-fits-all’ solution. Clinical evidence will bring us to the next step, which will lead to the next generation of devices.