Symptomatic peripheral arterial disease (PAD) patients undergoing recurrent lower extremity revascularisation have higher rates of ischaemic events, particularly acute limb ischaemia, than those patients who are undergoing their first peripheral revascularisation. This is the conclusion presented by Marc Bonaca (University of Colorado, Aurora, USA) at the 2020 meeting of the Cardiovascular and Interventional Radiological Society of Europe (CIRSE; 12–15 September, virtual). Bonaca and colleagues conducted a subgroup analysis from the VOYAGER PAD trial, which examined the efficacy and safety of Rivaroxaban in patients with PAD undergoing recurrent lower extremity revascularisation.
“We know from trials of patients with PAD that a history of a prior peripheral revascularisation is associated with a very high risk of acute limb ischaemia, even long-term years after the intervention was done,” Bonaca told online attendees. “More recent data from the landmark COMPASS trial shows that, in patients with chronic PAD, those who had a prior history of revascularisation are at major heightened risk of major adverse limb events.”
VOYAGER PAD is a trial of 6,564 patients with symptomatic lower extremity PAD undergoing peripheral revascularisation. Patients were randomised 1:1 in a double blind fashion to either the Rivaroxaban group, where they received 2.5mg of the drug twice daily, or a placebo group. The primary efficacy endpoint was a five-point composite of acute limb ischaemia, major amputation of vascular aetiology, myocardial infarction, ischaemic stroke, or cardiovascular death. The primary safety endpoint was thrombolysis in myocardial infarction (TIMI) major bleeding.
In an assessment of the primary efficacy endpoint, the rivaroxaban group performed better than the placebo group, with fewer incidences of the five major complications included in the composite measurement. “In spite of best medical therapy in this population [those patients treated with a placebo], the event rate was nearly 20%, which was extremely high—nearly one in five had an event,” Bonaca said. A Kaplan-Meier curve plotting cumulative incidence of the composite measurement of major adverse events against time since randomisation revealed that, at three-years post-treatment initiation, the event rate was 19.9% in the placebo group, and 17.3% in the rivaroxaban arm (hazard ratio [HR], 0.85; 95% confidence interval [CI], 0.76–0.96; p=0.009).
“Rivaroxaban risk-benefit was apparent early, and continued over time,” Bonaca commented, “with an absolute risk reduction of 2.6% at three years, and a number needed to treat of just 39.”
Against this backdrop of successful rivaroxaban use in the main VOYAGER PAD trial, Bonaca and colleagues conducted a subanalysis investigating symptomatic PAD patients undergoing recurrent lower extremity revascularisation versus those undergoing their first revascularisation. They hypothesised that repeat revascularisation patients would have a higher rate of acute limb ischaemia, and would derive “even greater benefits with a rivaroxaban plus aspirin strategy versus aspirin alone”.
The presence of known prior revascularisation was reported by investigators at baseline, and was defined as any history of endovascular, hybrid, or surgical lower extremity revascularisation. The primary outcome was the same composite as in the VOYAGER PAD study, and a COX model with interaction terms was used to assess for heterogeneity of efficacy and safety of rivaroxaban by prior lower extremity revascularisation status.
From a comorbidity perspective, the prior revascularisation group had a higher risk profile, Bonaca shared, with more hypertension, diabetes, and hyperlipidaemia. PAD characteristics were similar between the two cohorts, with the prior revascularisation group having a slightly lower risk: “they were more likely to be treated with an endovascular revascularisation, they had less frequently presented with acute limb ischaemia versus claudication, and the [average] ankle-brachial index was a bit higher. […] It is worth noting that they were very well-treated.”
In the placebo cohort, the cumulative event rate for those with no prior lower extremity revascularisation was 17.7%—“very high,” Bonaca pointed out, “even in this very well-treated population.” Nevertheless, those patients who had had a prior revascularisation had a higher cumulative event rate of 23.8%, representing a 6.1% increase in risk.
“When we look at the efficacy of Rivaroxaban, stratified in these two groups [prior revascularisation and no prior revascularisation], we see the benefit is there for both groups,” Bonaca explained.
In the Rivaroxaban cohort, those with no prior revascularisation had a cumulative event rate of 16.9%, while those who had undergone an earlier revascularisation had a cumulative event rate of 18.1%. The impact of prior revascularisation was therefore less in the Rivaroxaban cohort than in the placebo cohort.
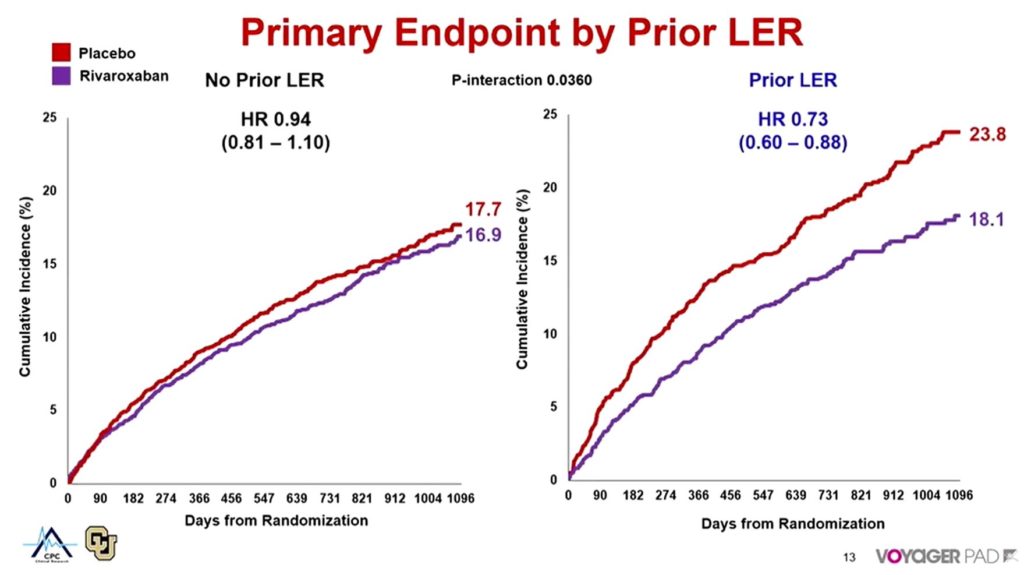
Comparing the placebo and rivaroxaban cohorts (Figure 1), Bonaca told delegates: “The benefit [of Rivaroxaban] appears even greater in those who had a prior history of revascularisation, with a HR of 0.73, and a trend towards heterogeneity, meaning there may be an even greater benefit in this high-risk group.”
Wondering what could be driving this difference, Bonaca and colleagues looked at the limb outcomes of acute limb ischaemia and vascular amputation in patients treated with Rivaroxaban with and without prior lower extremity revascularisation. In placebo patients with no prior revascularisation, rates of acute limb ischaemia and vascular amputation were lower at three-years post-randomisation than they were in the group of patients who had had previous revascularisation: 6% versus 10.8%, and 3.4% versus 4.5%, respectively.
As Bonaca put it: “Patients in the prior revascularisation group have a very high risk of acute limb ischaemia, almost 11%, and that is driving this risk-profile, and that benefit for acute limb ischaemia [with Rivaroxaban] is even greater in patients with a prior history of revascularisation. This does look like our hypothesis—that repeat revascularisation patients are at higher risk and would derive a greater benefit of Rivaroxaban—was met in this analysis.
“Rivaroxaban plus aspirin versus aspirin alone reduces irreversible harm events, so the heart, limb, and brain in patients that are undergoing revascularisation, but in this subgroup with prior revascularisation, there is a particularly robust benefit, and really notably in acute limb ischaemia. The current analysis demonstrates that within this population, those with multiple revascularisations are at higher risk than those who have undergone a first revascularisation only, and may derive particularly robust benefit from Rivaroxaban plus aspirin versus aspirin alone. These observations further demonstrate the heterogeneity of risk in the PAD population, and may assist in clinical risk stratification and therapeutic decision-making.”