 North Dallas Research Associates, Dallas, Texas, and its private practice, Cardiac Center of Texas, have announced their participation in the Tack Optimized Balloon Angioplasty II Below the Knee (TOBA II BTK) clinical trial.
North Dallas Research Associates, Dallas, Texas, and its private practice, Cardiac Center of Texas, have announced their participation in the Tack Optimized Balloon Angioplasty II Below the Knee (TOBA II BTK) clinical trial.
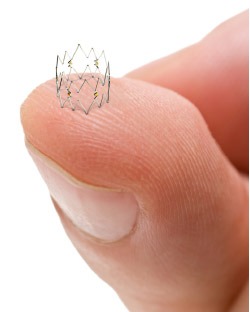
Using the Tack endovascular system (Intact Vascular), the technology is designed specifically to repair dissections following standard balloon angioplasty in the popliteal and tibial arteries with minimal stress to the vessels.
“We are very excited to enrol the first patient in Texas to be treated in this ground-breaking clinical study,” says M Akram Khan, cardiologist at Cardiac Center of Texas, Dallas, USA. “TOBA II BTK provides the latest vascular technology for those in the Dallas area suffering from advanced peripheral artery disease.”
The Tack endovascular system is equipped with self-sizing technology that allows one Tack implant to fit arteries ranging from 1.5mm to 4.5mm in diameter. This flexibility eliminates the need to precisely size the device to the arterial diameter, which is necessary with conventional stents. The device is then intended to allow physicians to “spot treat” the vessel only where dissections are present, rather than leave dissections untreated or covering them with large metal stents. According to a company release, this technique minimises the amount of implanted metal and reduces vessel trauma and inflammation.
“Advanced peripheral artery disease and critical limb ischaemia are large areas of fast-growing unmet need, both clinically and from a health economics perspective,” adds Khan. “Patients with critical limb ischaemia suffer from debilitating symptoms and have few effective treatment options. It is important for the medical community to collaborate in this study to further the treatment options for patients with such advanced disease.”