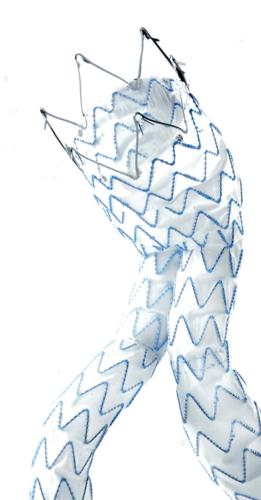
Medtronic has received CE mark for its Endurant II/IIs stent graft system to treat abdominal aortic aneurysm patients using a ChEVAR procedure—a parallel graft chimney technique that uses commercially available balloon expandable covered stents combined with a standard aortic stent graft.
This expanded indication in the EU enables the Endurant II/IIs stent graft system to be used in patients with complex aneurysms with short aortic neck lengths >=2 mm, expanded from the prior neck length indication >=10 mm.
“Treating aneurysm patients with short aortic necks has been a long-time challenge for clinicians performing endovascular aneurysm repair (EVAR) to treat abdominal aortic aneurysm patients,” says Giovanni B Torsello, chief of Vascular Surgery, St Franzkisus Hospital, Münster, Germany and co-author of the PROTAGORAS study. “The availability of a standardised approach which increases anatomical applicability will help establish a new standard for patients with complex forms of abdominal aortic aneurysm that may not have been suited for previous procedures.”
According to a company release, the CE mark is supported by a comprehensive review of clinical data from literature using the Endurant II/IIs stent graft system with the ChEVAR technique. In the flagship PROTAGORAS study, outcomes were tracked with radiologic follow up over a mean of two years. The study used a standardised procedural approach with the Endurant system and balloon expandable covered stents. The results, which were published in the Journal of Vascular Surgery, demonstrated that standardised use of the Endurant II/IIs stent graft system with ChEVAR in 128 patients is associated with 100% technical success, statistically significant aneurysm sac regression (p=0.001), 95.7% primary patency of the chimney grafts and a low incidence of chimney-related reinterventions.
The Endurant II/IIs stent graft system is based on Medtronic’s Endurant stent graft system. The original Endurant system received the CE mark in June 2008. The new expanded ChEVAR indication will be initially commercialised in Europe, and then in other countries that recognise the CE mark approval. In the US, Food and Drug Administration approval for the Endurant stent graft system was granted in December 2010. In the US, the Endurant II/IIs stent system is approved for neck lengths >=10mm and <=60° infra-renal angulation and it is not approved for this expanded indication.