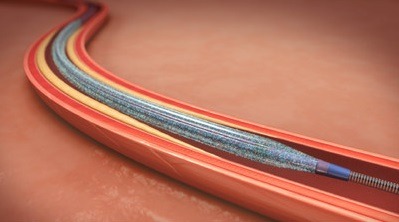
 Medtronic Canada, a subsidiary of Medtronic, has announced it has obtained Health Canada licence for the IN.PACT Admiral drug-coated balloon. IN.PACT Admiral is a primary endovascular device used in patients with peripheral artery disease in the upper leg, specifically, in the superficial femoral and popliteal arteries.
Medtronic Canada, a subsidiary of Medtronic, has announced it has obtained Health Canada licence for the IN.PACT Admiral drug-coated balloon. IN.PACT Admiral is a primary endovascular device used in patients with peripheral artery disease in the upper leg, specifically, in the superficial femoral and popliteal arteries.
Medtronic says that what distinguishes the IN.PACT Admiral from other alternatives for peripheral artery disease is the coating formulation, composed of a proven anti-proliferative drug (paclitaxel) and an excipient (urea). Once inflated, the coating comes into contact with water in the bloodstream, which hydrates the urea, facilitating the release of solid-phase paclitaxel to the vessel wall to help prevent restenosis.
“What many people do not realise is that peripheral artery disease in the legs is often connected to health conditions in other parts of the body, especially in the heart,” said Michael Blackwell, senior director of Medtronic’s Cardiovascular Group in Canada. “With drug-coated balloons, we now have a way to more effectively reduce plaque build-up caused by peripheral artery disease, and we are excited that hospitals in Canada are now able to offer this new technology.”
Supported by a “robust body” of evidence, a Medtronic press release notes that IN.PACT Admiral has been studied in more than 20 individual clinical trials, “demonstrating durable safety and clinical benefits”. Recently, three-year data from the IN.PACT SFA clinical trial demonstrated the highest rate of primary patency and lowest rate of clinically driven target lesion revascularisation in a pivotal study of interventional treatments for peripheral artery disease.
“Patients experiencing incapacitating intermittent claudication or rest pain need a safe and durable treatment option,” said Andrew Benko, Centre Hospitalier Universitaire de Sherbrooke, Sherbrooke, Canada. “In my practice, IN.PACT Admiral will be the device of choice for such patients with femoropopliteal lesions giving the opportunity for outstanding clinical results with very low reintervention rates. This technology has been one of the most significant advancements I have seen in the endovascular management of peripheral artery disease.”












