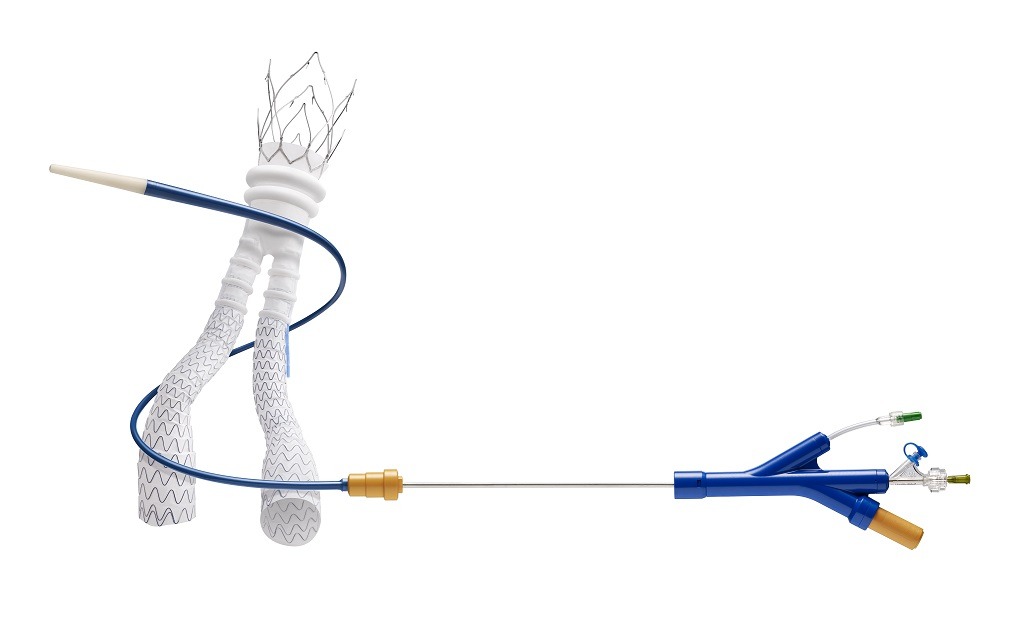
Clinical data presented at the 2016 VEITH symposium (New York City, USA) of the global Ovation Pivotal Trial has revealed positive five-year safety and effectiveness for the Endologix Ovation system.
The results were presented by Manish Mehta, director of Vascular Health Partners of Community Care Physicians PC (New York, USA), and the US principal investigator for the global trial.
The Ovation Pivotal Trial included a total of 161 patients, enrolled in Chile, Germany and USA from November 2009 to December 2011. Through five years, the key highlights from the data included:
- Broad patient applicability, with 40% of the patients treated outside the labelled indications of other endovascular aortic repair (EVAR) devices
- Stable aortic neck diameters with an average expansion of 0.1%, compared to 25% as reported with other EVAR devices
- Ninety-seven point five per cent freedom from secondary interventions related to type 1 endoleak
- No migration or conversions
Mehta comments, “The five-year data from the Ovation Pivotal Trial confirms the long-term safety and durability of the Ovation system. The study included the broadest range of abdominal aortic aneurysm (AAA) patients ever treated in an endovascular AAA investigational device exemption trial, and demonstrates the effectiveness of Ovation system’s ultra-low profile delivery system and polymer sealing technology.”













