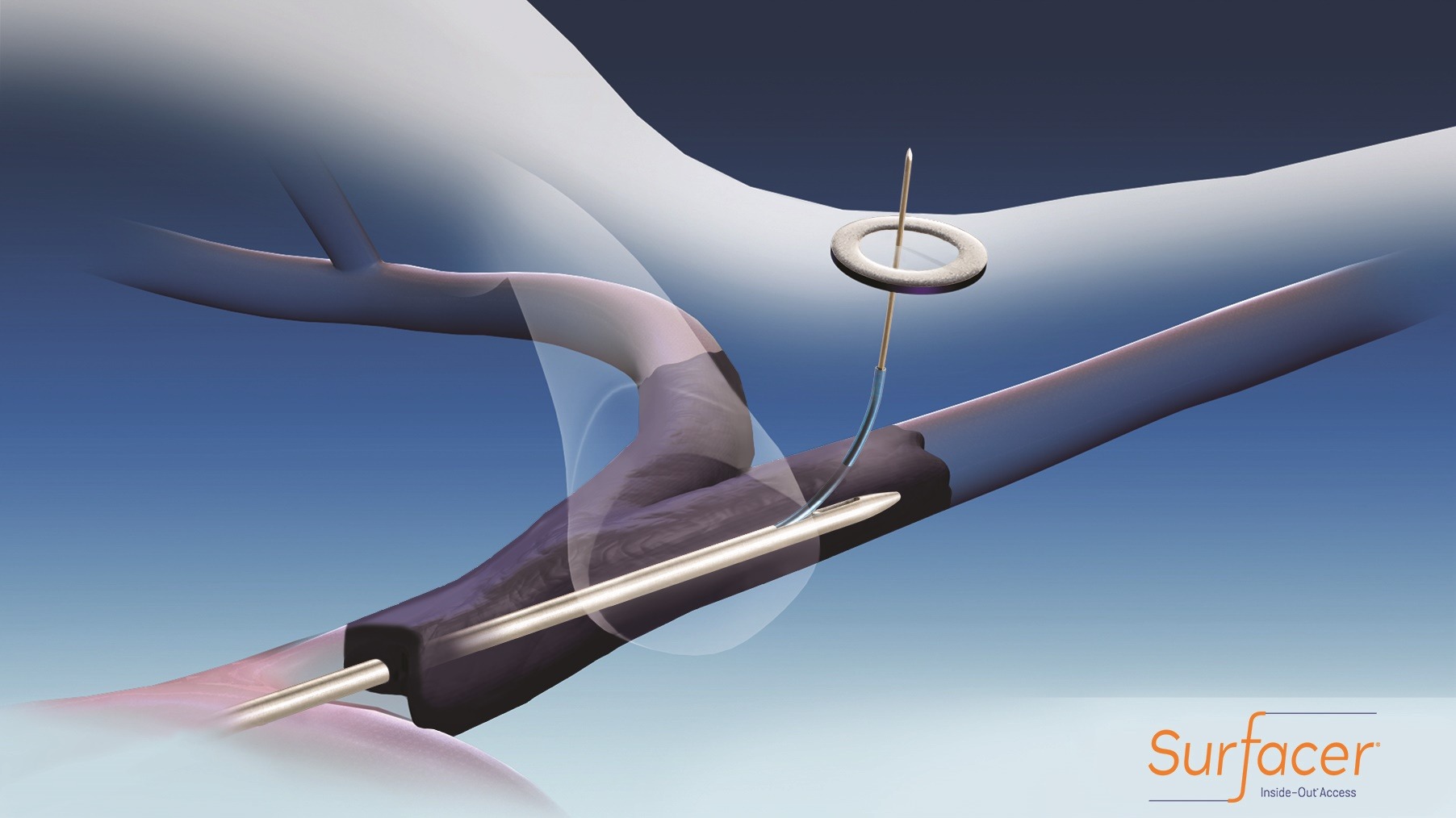
 Bluegrass Vascular Technologies has enrolled over one third of its patients in the company’s post-market SAVE (Surfacer System to Facilitate Access in Venous Occlusions) clinical study. The SAVE study is an international, prospective, multicentre clinical follow-up study designed to confirm clinical performance and safety of the Surfacer inside-out access catheter system, a device indicated for obtaining central venous access to facilitate catheter insertion into the central venous system in patients with chronically occluded veins.
Bluegrass Vascular Technologies has enrolled over one third of its patients in the company’s post-market SAVE (Surfacer System to Facilitate Access in Venous Occlusions) clinical study. The SAVE study is an international, prospective, multicentre clinical follow-up study designed to confirm clinical performance and safety of the Surfacer inside-out access catheter system, a device indicated for obtaining central venous access to facilitate catheter insertion into the central venous system in patients with chronically occluded veins.
“I look forward to presenting initial results of my experience in the SAVE study at the upcoming CX Symposium,” states Vladimir Matoussevitch, Vascular Surgeon at the University Hospital of Cologne in Cologne, Germany and one of the multicentre Investigators of the SAVE study. “The Surfacer system is an exciting new technology that offers my patients a promising option for reliable catheter placement during arteriovenous access creation.”
Bluegrass Vascular will be co-presenting with Merit Medical Systems during a lunch symposium on April 26th from 12:30–1:30pm. The event will be presented by Gürkan Sengölge, associate professor, interventional nephrologist at the University of Vienna, Vienna, Austria, and John Gurley, interventional cardiologist at the University of Kentucky Medical Center, Lexington, USA, and founder of Bluegrass Vascular.
The company secured CE mark approval of the Surfacer system in 2016 and recently announced its worldwide distribution relationship with Merit Medical.













