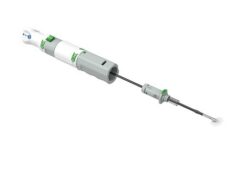
Essential Medical has received investigational device exemption (IDE) approval from the US Food and Drug Administration (FDA) to begin the US clinical trial of X-Seal, the company’s 6F vascular closure device.
The single-arm, pivotal study will consist of 180 patients at 10–15 sites throughout the USA, Canada, and the EU. The safety and efficacy of the X-Seal device will be evaluated through a comparison of measured complication rate, time to haemostasis, and time to ambulation against a performance goal.
Gary Roubin, chief medical officer of the company, stated, “The X-Seal device provides a necessary improvement to current closure devices. Combining proven technology with novel deployment control features, the X-Seal device allows for consistent closure performance. I am pleased that the device is one step closer to US commercial availability.”
The X-Seal 6F vascular closure device is already CE-marked with a post-market study completed in the EU in February 2016 with excellent results. This X-Seal IDE approval compliments the IDE approval recently secured for company’s Manta large bore closure device.