Essential Medical has announced that it has completed a series B financing of US$14.9 million. The round was led by Amzak Health along with original series A investors including DSM Venturing, the venture investment arm of Royal DSM.
Greg Walters, president and CEO of the company, stated, “We are thrilled that Amzak Health has the confidence in the novel Essential Medical technology and our team to lead this round of financing along with our original investors. The Amzak group will also provide additional expertise and experience to our board of directors as our company transitions to commercialisation in Europe and the initiation of the US clinical trial for our Manta product.”
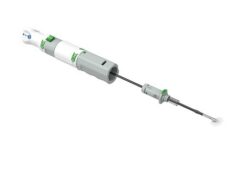
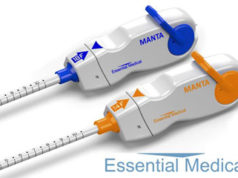
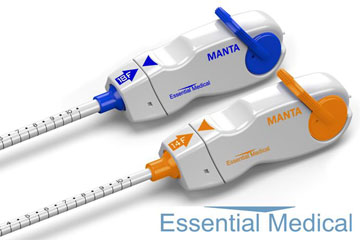
Manta is a novel CE-marked vascular closure device designed to close punctures ranging from 10F to 24F at femoral arterial access sites after cardiac catheterisation procedures such as transcatheter aortic valve implantation (TAVI), endovascular treatment of abdominal aortic aneurysms (EVAR), ventricular assist (VAD), and balloon aortic valvuloplasty (BAV). According to Essential Medical, these procedures are the fastest growing segment of the cardiovascular market and are driving the potential worldwide market for large bore vascular closure devices to exceed US$600 million within five years. Closure of large bore femoral access sites has been associated with significant morbidity including long times to achieve haemostasis, extended procedure time, need for a vascular surgeon in the catheterisation lab, delayed ambulation, higher rate of complications and higher total cost of care. Manta was designed to address the complexities of closing large punctures in high-pressure vessels utilising novel closure technology. Manta’s fail-safe deployment provides immediate haemostasis in order to reduce complications associated with large bore closure.