This advertorial is sponsored by Shape Memory Medical.

A wide variety of vessels treated with no device-related adverse events is a key recent finding to emerge from the EMBO postmarket surveillance (EMBO-PMS) registry.
EMBO-PMS is a prospective, multicentre registry of the Impede, Impede-FX, and Impede-FX RapidFill embolisation plug systems (Shape Memory Medical) when used for peripheral vascular embolisation. The study, which is enrolling up to a combined 125 patients at four centres in the UK and eight in Germany, is evaluating the use of these shape memory polymer-based devices in patients with peripheral vascular disease and their potential to embolise the target area after deployment. Patients are being followed at 90 days and one year.
Robert Morgan (St George’s University Hospitals NHS Foundation Trust, London, UK), co-principal investigator for EMBO-PMS, recently shared one-year outcomes from the trial at the 2024 VEITHsymposium (19–23 November, New York, USA).
During his presentation, Morgan highlighted the imaging clarity offered by the Impede devices, emphasising the minimal imaging artifact postimplantation compared to embolisation with coils, which can produce significant artifact on computed tomography (CT) imaging.
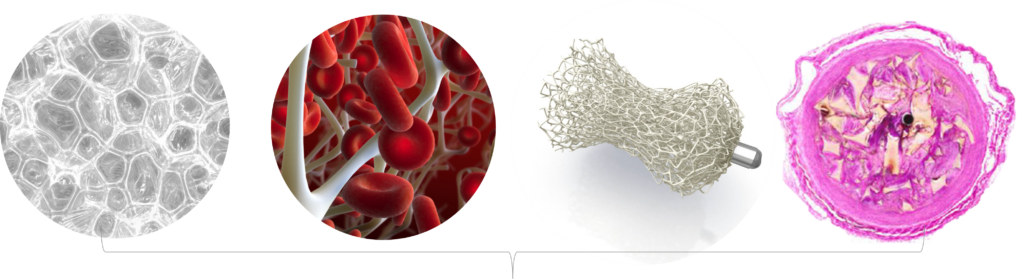
Morgan continued that the radiolucent shape memory polymer incorporates a porous polyurethane embolic scaffold that promotes rapid thrombosis followed by collagen ingrowth as shown in preclinical studies.
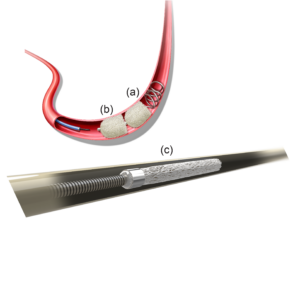
Elaborating on how the shape memory polymer devices are deployed during a procedure, Morgan explained: “The plugs are crimped for catheter delivery to the target vessel and self expand once exposed to blood for embolisation.”
Regarding demographics, Morgan detailed that, to date, 82 patients have been treated within this study, with 92 vessels embolised. Continuing, Morgan stated that of greatest significance were the variety of vessels treated, which included intercostal arteries, ovarian veins, iliac arteries, and inferior mesenteric arteries. “We see broad utility across multiple applications for standalone use and in combination with other embolic devices,” the presenter added.
Following his presentation of the results to date, Morgan commented that the data illustrate the safety and efficacy of novel shape memory polymer embolic devices for embolisation of peripheral vasculature and that further experience and longer-term follow-up will demonstrate the utility and durability of the devices. To date, no device-related SAEs have been reported, and 90-day follow-up has been completed in over half of the 82 patients enrolled with a high rate of sustained occlusion.
Morgan also referenced the initial use of these shape memory polymer devices for peripheral vascular embolisation described by Holden et al, reporting no recurrent clinical symptoms attributable to treated vessel embolisation or recanalisation through up to four-year follow-up.
Shape Memory Medical notes that it continues to study the use of its shape memory polymer devices in other clinical applications outside the scope of their approved indications for use, including the Impede- FX RapidFill device for active aortic aneurysm sac management in the AAA-SHAPE randomised controlled pivotal trial.
Disclaimers:
Robert Morgan is co-principal investigator of the EMBO-PMS study. For more information about the EMBO-PMS study, please visit https:// clinicaltrials.gov/study/NCT04044443?term=EMBOPMS& rank=1.
Not all devices discussed in this interview are available in all regions. The content contains information about the AAA-SHAPE pivotal trial, Shape Memory Medical’s prospective, multicentre, randomised, open-label trial of the Impede-FX RapidFill when used for prophylactic abdominal aortic aneurysm (AAA) sac filling during elective endovascular aneurysm repair (EVAR). For more information about the AAA-SHAPE study, please visit https://clinicaltrials.gov/study/NCT06029660.
In countries recognising CE marking, the Impede embolisation plug, the Impede-FX embolisation plug, and Impede-FX RapidFill are indicated to obstruct or reduce the rate of blood flow in the peripheral vasculature. In the USA, the Impede embolisation plug is indicated to obstruct or reduce the rate of blood flow in the peripheral vasculature and the Impede-FX embolisation plug is indicated for use with the Impede embolisation plug to obstruct or reduce the rate of blood flow in the peripheral vasculature.
The Impede-FX RapidFill device is not approved for sale in the USA or Japan. Data on file at Shape Memory Medical.