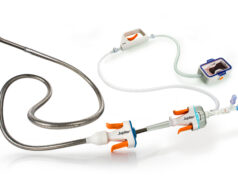
Truvic Medical, a wholly owned subsidiary of Imperative Care, announced in a press release that it has received 510(k) clearance from the US Food and Drug Administration (FDA) for the Prodigy thrombectomy system, designed for the treatment of peripheral vascular thrombus.
Truvic Medical, a wholly owned subsidiary of Imperative Care, announced in a press release that it has received 510(k) clearance from the US Food and Drug Administration (FDA) for the Prodigy thrombectomy system, designed for the treatment of peripheral vascular thrombus.
“We are excited to receive FDA clearance for our first thrombectomy system. We look forward to working with experts in the field to translate Prodigy’s novel design elements and features into superb clinical outcomes,” said Mike Buck, CEO of Truvic. “This represents our first regulatory milestone as part of our strategy to advance multiple programmes designed to meet patients’ needs and bring more innovative technologies to the market, faster.”
“Imperative Care and Truvic share a culture of innovation and intense commitment to the needs of patients. I am pleased that these common values continue to help propel our development programmes towards elevation of patient care,” Fred Khosravi, chairman and CEO of Imperative Care, added.
Truvic is based in Campbell, California, USA and its technology is designed to redefine peripheral vascular thrombus management by enabling single-session thrombus removal without the use of thrombolytics.
Imperative Care is a medical technology company focused on developing the next generation of solutions to address the vast and urgent needs in stroke intervention and peripheral thrombectomy. The company has two FDA 510(k) cleared product platforms, as well as an expansive stroke technology development pipeline.