This advertorial is sponsored by Bentley.

There are several covered stents on the market for both fenestrated endovascular aneurysm repair (FEVAR) and branched endovascular aneurysm repair (BEVAR). However, all are used off-label for these procedures, and there is currently no consensus about when to use which stent. Bentley (Hechingen, Germany) is investing heavily to obtain the necessary evidence for these two indications. The company recently revealed that the first patient has been recruited into a trial investigating the BeGraft PLUS as a dedicated bridging stent in BEVAR for the treatment of complex aortic aneurysms. According to Bentley, it is the first study of its kind worldwide.
Seeking expert knowledge and opinion on the subject, Vascular News spoke to the coordinating investigator of the BEVAR study, Professor Martin Austermann (St Franziskus Hospital, and University of Münster, Münster, Germany). Austermann detailed his extensive experience with FEVAR and BEVAR procedures, his excitement that the BEVAR trial will bring much-needed evidence into the equation, and his belief that solid data will move the field closer to realising an ideal bridging stent.
When did you start implanting fenestrated and branched endografts?
I started implanting fenestrated and branched endografts around 2008, and have been performing a respectable number of these procedures since around 2010. I now do about 60–80 cases per year.
What are your main findings since 2008?
Over time, I have become a big fan of branched endografting, and have developed a technique that is very quick and highly standardised. I have been able to train some other colleagues so that we have a very standardised process for every repair.
When you consider fenestrations, you often need customisation. You have to plan the case exactly and if there are any mistakes during this phase, you can encounter serious problems during implantation. With branches, sometimes you need customisation—for example, if the patient only has one renal artery—but in most cases you can use a standardised device. Over time, I have been performing more and more thoracoabdominal procedures with branches, for example, and I tend to reserve the fenestrated technique only for juxtarenals, or infrarenals with short necks.
What kind of bridging stent grafts do you prefer for branches and why?
Over the last decade, I can categorise my use of these devices into three phases that have helped me to determine the kind of bridging stent I prefer for branches. In the first phase, I used a stainless steel stent that has very exact placement and is highly visible, but it is not very flexible. Therefore, when we used this stent, we would do a relining with a self-expanding bare metal stent. This works very well in the superior mesenteric artery (SMA) and celiac trunk, but not very well in the renal arteries. I would say we had problems in about 10% of renal arteries, which is too many!
I then switched to a self-expanding stent for the renal arteries. This works well, but is not very accurate to deploy and is not visible. Therefore, we would do a combination of the balloon-expandable stent and an extension with the this self-expanding stent to help flexibility at the distal edge. This combination also worked very well in the renals, but brought additional cost and made the procedure more complex.
The last phase began when a stainless steel stent with a PTFE (polytetrafluoroethylene) hold came on to the market that I thought was a very good, especially in terms of flexibility. I thought for the first time that maybe this is the solution for the renal arteries in branched endografting. However, we had a very severe learning curve and I would say it is not the best solution for every renal artery.
In 2017, Bentley developed the BeGraft PLUS, which has a “stent-in-stent” design comprising two layers of expanding polytetrafluoroethylene (ePTFE). One of the main features is the kink resistance. If you compare the previous stent with the BeGraft PLUS, you might have more flexibility with the former, but if you have an angle in the anatomy, it can develop a kink, which can lead to an occlusion. Flexibility is not the only factor, you must have a stent that is kink resistance and has the right radial force, and these are both features of the BeGraft PLUS.
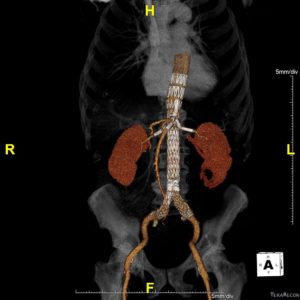
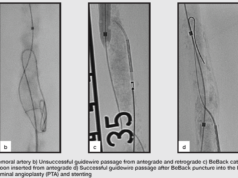
Courtesy Dr Michel Bosiers (Münster, Germany)
Can you tell us about the BEVAR study and what you hope it will achieve?
The study aims to carry out 100 BEVAR procedures in patients seeking elective repair of thoracoabdominal aortic aneurysm (TAAA).
The primary efficacy endpoint is comprised of a measure of technical success defined as the successful introduction and deployment of the BeGraft PLUS implanted as a bridging stent in BEVAR, with bridging stent patency at 12 months (absence of restenosis (≥50% stenosis) or sole target vessel occlusion based on computed tomography (CT) angiography at 12 months. The primary safety endpoint at 12 months relates to the absence of procedure-related complications and bridging stent-related endoleaks. Enrolment is estimated to take approximately 12 months, and total follow-up will be 24 months.
Study participants include many experts in the endovascular field, including Professor Eric Verhoeven (Nuremberg Hospital South, Nuremberg, Germany), Professor Nikolaos Tsilimparis (Ludwig-Maximilians University Hospital, Munich, Germany), Professor Tilo Kölbel (University Hospital Eppendorf, Hamburg, Germany), Professor Dierk Scheinert and Dr Andrej Schmidt, Park Hospital, Leipzig, Germany), Professor Karin Pfister (University Hospital Regensburg, Regensburg, Germany), Professor Drosos Kotelis (University Hospital Aachen, Aachen, Germany), and Professor Phillipp Geisbüsch (Hospital Stuttgart, Stuttgart, Germany).
I am very excited to learn more about these procedures, especially regarding the renal arteries, because the renal artery connection is always the Achilles’ heel of this repair. In the past, I used the BeGraft PLUS only in certain cases, but now the study has been initiated, I try to use it very liberally so as to learn about this stent in relation to others. My impression of the BeGraft PLUS is positive, but we have no studies, like with all the other stents, so we need these data.