Zinc ferrite (ZnFe2O4) nanoparticles have the potential to be used as a future antimicrobial and wound-healing drug, a recent study in the Journal of Nanobiotechnology reports. Lead author Reihaneh Haghniaz (University of California Los Angeles [UCLA], Los Angeles, USA) and colleagues conclude: “The prepared ZnFe2O4 nanoparticles presented excellent biocompatibility and haemocompatibility with human dermal fibroblast cells and human red blood cells, and exhibited potent antimicrobial activity against both Gram-positive and Gram-negative microbial strains at biocompatible concentration.”
Contextualising their research, Haghniaz et al explain the clinical need for new anti-bacterial therapies: “Increasing antibiotic resistance continues to focus on research into the discovery of novel antimicrobial agents. Due to its antimicrobial and wound healing-promoting activity, metal nanoparticles have attracted attention for dermatological applications. This study is designed to investigate the scope and bactericidal potential of zinc ferrite nanoparticles, and the mechanism of anti-bacterial action along with cytocompatibility, haemocompatibility, and wound healing properties.”
They explain their choice of nanoparticle: “Among different metal nanoparticles, iron oxide-based nanoparticles, especially spinel ferrites, have recently emerged as popular candidates for emerging biomedical applications due to their chemical stability, biocompatibility, the reasonable cost compared to the other metal nanoparticles, such as silver nanoparticles (i.e., gold standard anti-bacterial nanoparticles), and their unique ferromagnetic properties.”
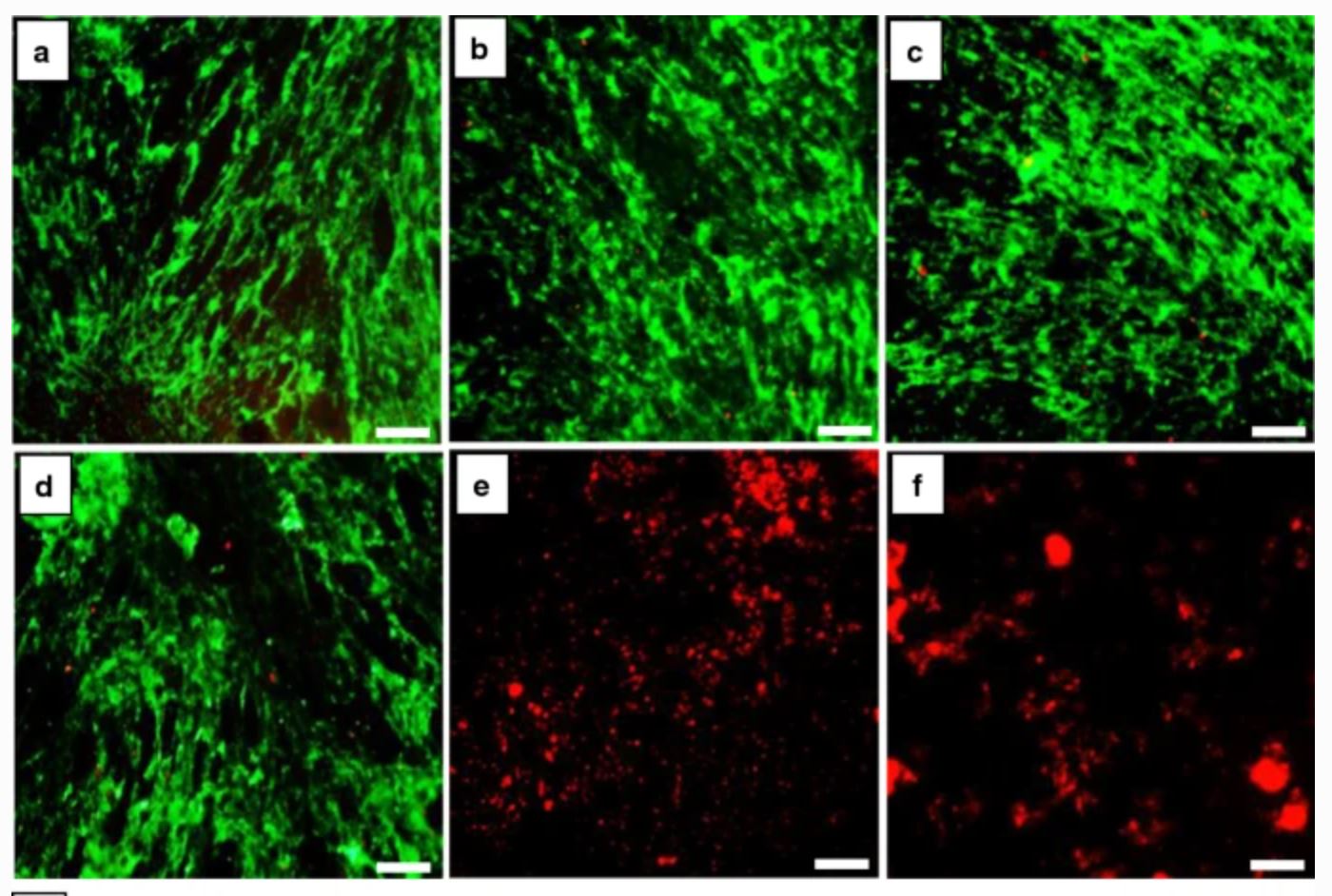
Following the synthesis of the ZnFe2O4 nanoparticles, the investigators conducted a cytocompatibility study to evaluate their cytotoxicity. Haghniaz et al performed a live/dead viability assay to analyse cellular status (alive or dead) according to the integrity of the plasma membrane, as well as a PrestoBlue assay that can analyse mitochondrial activity.
The live/dead viability assay showed that ZnFe2O4 nanoparticles exhibited dose-dependent toxicity to the human dermal fibroblast cells. At low nanoparticle concentrations (0–250 µg/mL), no cytotoxic effects were observed in the human dermal fibroblast cells, and the majority of cells were stained green, signifying their living status. Whereas, at a nanoparticle concentration of 500µg/mL and 1,000µg/mL, high numbers of red fluorescence were observed, denoting significant cell death.
Commenting on these findings, the authors write: “Through the live/dead assay, we were able to observe numerous live cells at doses of ≤125µg/mL, but to confirm that the live cells are not in a state of inhibition of cell proliferation, we further performed the PrestoBlue assay. Similar to the live/dead viability assay, at high nanoparticle concentrations of 500 and 1,000µg/mL, the metabolic activity of cells was significantly reduced by 80 % and 95 %, respectively. On the other hand, the metabolic activity of 62, 125, and 250µg/mL of ZnFe2O4 nanoparticles remained at 96%, 92%, and 84% respectively on day five of treatment. Based on these results, we confirmed that a safe dose of ZnFe2O4 nanoparticles for human dermal fibroblast cells was less than 125µg/mL, as at this concentration more than 90% of the cells were viable and metabolically active.”
In addition, a haemocompatibility assay revealed that the nanoparticles do not significantly rupture red blood cells up to a dose of 1,000µg/mL.
“Interestingly,” Haghniaz and co-authors say, “nanoparticles showed antimicrobial activity through multiple mechanisms, such as cell membrane damage, protein leakage, and reactive oxygen species generation, and were more effective against gram-positive bacteria. Furthermore, in vitro scratch assay revealed that ZnFe2O4 nanoparticles improved cell migration and proliferation of cells, with noticeable shrinkage of the artificial wound model.”
Due to these positive results, they conclude: “The optimised ZnFe2O4 nanoparticles can be used as an alternative therapeutic agent against drug-resistance microbial pathogens and also as a bandage to enhance burn-wound healing in near future”.
They suggest that future work should focus on investigating the short-term and long-term toxicity and wound-healing effect of ZnFe2O4 nanoparticles in animal models.













