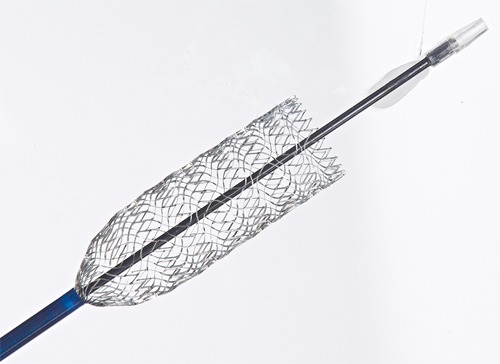
Veniti has closed on US$25m in Series D equity financing from Boston Scientific Corporation. The funds will allow Veniti to complete the VIRTUS trial and regulatory filing for the Vici Venous Stent system. The trial is being performed under a US Food and Drug Administration (FDA) Investigational Device Exemption (IDE). The company also intends to expand product development and commercial operations.
“We are extremely pleased to be partnering with such a high quality industry leader to advance our core technology, the Vici Venous Stent,” said Jeff Elkins, president and chief executive officer of Veniti. “This financing will allow us to complete a number of critical milestones and support more physicians treating patients suffering from venous outflow obstruction around the world.”
Citigroup Global Markets Inc acted as exclusive financial advisor to Veniti in connection with the transaction.