Positive single-centre 24-month results from Intact Vascular’s TOBA (Tack optimised balloon angioplasty) clinical study were presented at the 2017 VEITHsymposium 2017 (14–18 November, New York City, USA) by Christian Wissgott (Westküstenklinikum Heide, Heide, Germany).
The TOBA study enrolled 138 subjects at 13 sites in Europe. All study participants were suffering from peripheral artery disease caused by blockages in the superficial femoral or popliteal arteries, located in the upper part of the leg. All participants underwent percutaneous balloon angioplasty (PTA) and repair of any dissections (or tears) resulting from PTA using the Tack endovascular system.
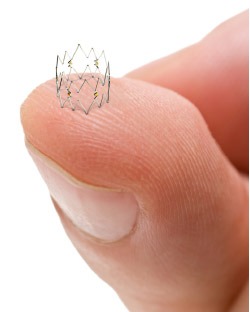
The Tack implant is a device for precision dissection repair following balloon angioplasty. The system is designed to help maintain vessel integrity and maximise blood flow to promote healing, improve outcomes and save limbs. The system leaves a minimal amount of metal in the artery, reduces mechanical stress on the arterial wall and preserves future treatment options, Intact Vascular says. Unrepaired dissections—which are frequent following PTA procedures—increase the probability of acute arterial occlusion and may continue narrowing the artery, which leads to lower long-term patency (or openness) rates.
Wissgott’s single-centre 24-month Kaplan-Meier patency results were unchanged from the favourable twelve-month rate of 87.5%. “The Tack implant offers a new paradigm for focal dissection repair in the superficial femoral or popliteal arteries,” Wissgott said. “Zero reduction in vessel patency over twelve months appears to be attributable to the Tack design, which leaves 70–80% less metal behind when compared to traditional stents. In addition, the Tack’s lower chronic outward force minimises vessel inflammation and reduces vessel trauma that can contribute to restenosis.”
“We are very pleased with Christian Wissgott’s 24-month single-centre results from our TOBA study,” said Bruce Shook, Intact Vascular’s president and CEO. “The results illustrate the potential for improvement of long-term patency following angioplasty with precision dissection repair using the Tack endovascular system.”
Intact Vascular is sponsoring three clinical trials to evaluate its Tack system: TOBA II, TOBA II BTK and TOBA III. TOBA II is investigating the combination of the Tack device with both plain and drug-coated balloon angioplasty in the arteries above the knee, and completed enrolment in February 2017. TOBA II BTK is investigating the combination of the Tack device with plain balloon angioplasty in the arteries below the knee and is actively enrolling patients. TOBA III is currently underway in Europe and is investigating the combination of the Tack device with drug-coated balloon angioplasty; enrolment is nearly complete.