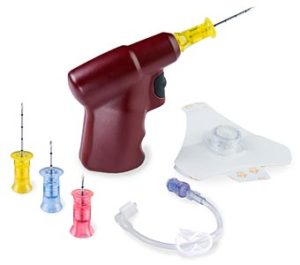
Teleflex has announced it has received 510(k) clearance from the US Food and Drug Administration to expand the indications for use of the Arrow EZ-IO intraosseous vascular access system. This device can be used when intravenous access is difficult or impossible to obtain in emergent, urgent, or medically necessary cases.
The Arrow EZ-IO system is now available with the expanded indication stating use of the device may be extended for up to 48 hours when alternate intravenous access is not available or reliably established in adults, and in paediatric patients 12 years and older.
“Vascular access is one of the most basic, yet critical, components of patient care,” said Michelle Fox, corporate vice president and chief medical officer, Teleflex. “The ability to use the EZ-IO system for a longer dwell time provides clinicians the option to utilise intraosseous (IO) access for the entire duration of therapy in patients with difficult vascular access where therapy is required for up to 48 hours.”
In patients who require longer-term access, the expanded indication gives clinicians additional time to establish vascular access safely, choosing the appropriate device and optimal site of insertion to meet the patient’s clinical needs. “These benefits are of particular importance in a time of constrained resources and patient surge,” said Fox.













