Tag: US FDA
Boston Scientific recalls Carotid Wallstent Monorail devices over “manufacturing defect”
Boston Scientific has recalled its Carotid Wallstent Monorail endoprosthesis owing to a “manufacturing defect” that has led to devices having an inner lumen that...
Aquedeon Medical receives US FDA approval to expand clinical trial of...
The US Food and Drug Administration (FDA) has approved the expansion of Aquedeon Medical's investigational device exemption (IDE) clinical trial evaluating the Duett vascular graft...
US FDA announces plans to address medical device shortage risks
The US Food and Drug Administration (FDA) has issued a statement outlining measures to enhance protections against medical device shortages.
According to Michelle Tarver,...
US FDA issues first draft guidance for AI-based medical device development
The US Food and Drug Administration (FDA) has issued draft guidance that includes recommendations to support development and marketing of safe and effective artificial...
Vascular News’ top 10 most popular stories of June 2024
The top 10 most-read Vascular News stories in June featured two US Food and Drug Administration (FDA) investigational device exemption (IDE) approvals, Boston Scientific's...
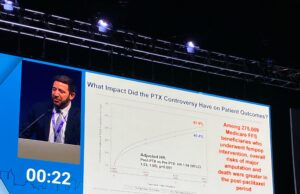
CX 2024: Paclitaxel device restrictions “did cause harm”
“Unfortunately, we are doing worse for our patients today,” were the sobering thoughts of Eric Secemsky (Boston, United States) during a late-breaking presentation in...
Shape Memory Medical advances endovascular embolisation by completing $38 million in...
Shape Memory Medical has announced that it has completed $38 million in Series C financing, which has been led by Earlybird Venture Capital, along...
TCT 2023: First presentation of complete patient-level dataset on paclitaxel and...
Data from a patient-level meta-analysis—a factor in the US Food and Drug Administration’s (FDA) recent change of position on the use of paclitaxel-coated devices...
FDA update: Paclitaxel-coated devices for PAD cleared of excess mortality risk,...
This advertorial is sponsored by Boston Scientific.
Data on hundreds of thousands of patients who have received lower limb endovascular treatment provide confidence in paclitaxel...
Vascular News’ top 10 most popular stories of July 2023
July's top 10 highlights the US Food and Drug Administration's long-awaited update on paclitaxel-coated devices to treat peripheral arterial disease, an interview with Rachel...
CIRSE supports use of paclitaxel-coated devices in femoropopliteal disease treatment
In June 2023, the Cardiovascular Interventional Radiological Society of Europe (CIRSE) published an editorial in Cardiovascular Interventional Radiology (CVIR). Written on behalf of CIRSE’s...
Timeline: Key milestones in the paclitaxel story
The release of a letter from the US Food and Drug Administration (FDA) concerning the use of paclitaxel-coated devices for the peripheral arterial disease...
FDA removes red flag for paclitaxel-coated devices after review finds data...
In a letter to healthcare providers dated 11 July 2023, the US Food and Drug Administration (FDA) communicates that the risk of mortality associated...
Swiss parliament votes to accept US FDA-approved medical devices
The Swiss Federal Assembly has voted in favour of accepting medical devices with US Food and Drug Administration (FDA) marketing authorisation in Switzerland.
A motion...
Biotronik’s Pulsar-18 T3 peripheral self-expanding stent system receives FDA approval
Biotronik recently announced that it has received US Food and Drug Administration (FDA) approval of its Pulsar-18 T3 peripheral self-expanding stent system. Full US...
New long-term data of paclitaxel devices continue to show no increased...
New long-term data from the SAFE-PAD (Safety assessment of femoropopliteal endovascular treatment with paclitaxel-coated devices) study were presented today as late-breaking clinical research at...
Cook Medical receives FDA Breakthrough Device designation for Zenith Thoraco+ endovascular...
Cook Medical’s Zenith Thoraco+ endovascular system (Thoraco+) has received Breakthrough Device designation from the US Food and Drug Administration (FDA), a press release reports....
FDA advisory panel issues recommendations on lifelong surveillance and long-term postmarket...
The US Food and Drug Administration (FDA) recently announced it has issued a letter to healthcare providers emphasising the importance of lifelong surveillance, including...
FDA issues two final guidances for including patient perspectives in medical...
The US Food and Drug Administration (FDA) has issued two final guidances providing recommendations for including patient perspectives in medical device clinical studies.
As...
FDA clears 12 new XO Cross microcatheters
Transit Scientific has announced US Food and Drug Administration (FDA) clearance of new hydrophilic-coated XO Cross microcatheters for guidewire support, exchange, and contrast media...
Sanford invents Breakthrough Device for vascular disease
An investigational device invented at Sanford Health (Sioux Falls, USA) that helps high-risk vascular disease patients has been granted a Breakthrough Device designation by...
BD announces 510(k) clearance of expanded indications for the Rotarex atherectomy...
BD recently announced it has received 510(k) clearance for expanded indications from the US Food and Drug Administration (FDA) for the Rotarex atherectomy system.
The...
Terumo Aortic announces first commercial implants of RelayPro endovascular device in...
Following the recent approval by the US Food and Drug Administration (FDA) of the RelayPro thoracic stent graft system for the treatment of patients...
FDA clears Koya Medical’s Dayspring compression system for lower extremities
Koya Medical announced today that it has received US Food and Drug Administration (FDA) 510(k) clearance for its active compression therapy system Dayspring for...
FDA clears Koya Medical’s Dayspring compression system for lower extremities
Koya Medical announced today that it has received US Food and Drug Administration (FDA) 510(k) clearance for its active compression therapy system Dayspring for...
FDA clears Koya Medical’s Dayspring compression system for lower extremities
Koya Medical announced today that it has received US Food and Drug Administration (FDA) 510(k) clearance for its active compression therapy system Dayspring for...
Flex Vessel Prep system receives new indication to address in-stent restenosis
VentureMed Group recently announced that the US Food and Drug Administration (FDA) cleared the company's Flex Vessel Prep system for use in the treatment...
Vascular surgeons encouraged to consult talking points document on paclitaxel devices
Vascular surgeons are being encouraged to take consideration of a set of talking points about the risks and benefits of paclitaxel-equipped devices—which has developed...
FDA grants Endospan IDE approval to initiate Nexus study
The US Food and Drug Administration (FDA) has granted Endospan an investigational device exemption (IDE) to start the TRIOMPHE study on the Nexus aortic...
Surmodics receives FDA 510(k) clearance for Sublime radial access 0.014 RX...
Surmodics recently announced it has received US Food and Drug Administration (FDA) 510(k) clearance for its Sublime radial access 0.014 rapid exchange (RX) percutaneous...
Merit Medical’s Wrapsody System receives CE mark
Merit Medical has received CE mark approval for the Wrapsody endovascular stent graft system from the British Standards Institution. The Wrapsody system is a...
FDA clears Aspire MAX mechanical thrombectomy system
Control Medical Technology has announced that the US Food and Drug Administration (FDA) has cleared its Aspire MAX 7 – 11F mechanical thrombectomy...
Terumo Aortic announces US FDA breakthrough device designation for Thoraflex Hybrid...
Terumo Aortic today announced that the US Food and Drug Administration (FDA) has granted breakthrough device designation for its Thoraflex Hybrid stented device for...
FDA grants Nexus aortic arch stent graft system breakthrough designation
Endospan was recently granted breakthrough device designation from the US Food and Drug Administration (FDA) for the Nexus aortic arch stent graft system.
The FDA’s...
Okami Medical announces first patients treated with Lobo vascular occluder
Okami Medical has announced the successful completion of the first cases with the Lobo vascular occlusion system. The first offering in the company's product...
FDA grants de novo clearance to Bluegrass Vascular for Surfacer system
Bluegrass Vascular Technologies (Bluegrass Vascular) announced today that the US Food and Drug Administration (FDA) has granted a de novo classification order for its...
Rist Neurovascular radial artery catheter receives FDA 510(k) clearance
Rist Neurovascular recently announced that it has received US Food and Drug Administration (FDA) 510(k) clearance to market the Rist Cath Radial Access Long...
Merit Medical’s EmboCube embolisation gelatin and Torpedo gelatin foam indicated for...
Merit Medical Systems has announced that both its EmboCube (syringe-loaded embolisation gelatin foam) and Torpedo (uniform, preshaped gelatin foam loaded into a cartridge with...
Intact Vascular expands Tack Endovascular System portfolio offering
Intact Vascular has announced US Food and Drug Administration (FDA) pre-market approval (PMA) for the expansion of its Tack Endovascular System (6F) portfolio. The...
Ra Medical Systems receives FDA IDE approval to begin pivotal atherectomy...
Ra Medical Systems has announced approval from the US Food and Drug Administration (FDA) that the company has provided sufficient data to support initiating...
MedAlliance receives third FDA breakthrough device designation for its sirolimus DEB...
MedAlliance, the first drug-eluting balloon (DEB) company in the world to receive US Food and Drug Administration (FDA) breakthrough device designation status for a...
FDA grants breakthrough device designation to Reflow Medical’s Temporary Spur stent...
Reflow Medical announces that the Temporary Spur stent system, a novel retrievable stent technology intended for the treatment of below-the-knee (BTK) peripheral arterial disease,...
Reaction to the Katsanos et al meta-analysis
Internal BIBA MedTech data show that perceptions on paclitaxel devices have shifted considerably in the twelve months that have elapsed since Konstantinos Katsanos (Patras,...
Merit Medical receives FDA breakthrough device designation for WRAPSODY endovascular stent...
Merit Medical Systems recently announced that it has been granted Breakthrough Device Designation by the US Food and Drug Administration (FDA) for the Merit...
Medtronic DCB receives US FDA approval to treat arteriovenous fistula lesions
Medtronic today announced US Food and Drug Administration (FDA) approval of the IN.PACT AV drug-coated balloon (DCB), a paclitaxel-coated balloon indicated for the treatment...
Update on regulatory landscape points to benefits of global collaboration
At the 2019 Vascular Interventional Advances (VIVA) conference in Las Vegas, USA (4–7 November), Misti Malone (US Food and Drug Administration Office for...
Okami Medical announces FDA 510(k) clearance and patent for the LOBO...
Okami Medical today announced 510(k) clearance by the US Food and Drug Administration (FDA) and initial launch of the LOBO vascular occlusion system.
The company...
Medtronic announces Shonin approval and launch of the Valiant Navion thoracic...
Medtronic today announced Shonin approval from the Ministry of Health, Labour and Welfare (MHLW) and the launch of the Valiant Navion thoracic stent graft...
Scott Gottlieb resigns as head of US FDA
The commissioner of the US Food and Drug Administration (FDA), Scott Gottlieb, has unexpectedly resigned after serving just shy of two years in the...
US government shutdown disrupting FDA work
The US government shutdown means the country’s Food and Drug Administration (FDA) cannot accept new user fees, which means the agency cannot accept new...
First patients treated in United States with OrbusNeich Teleport Microcatheter
The first patients in the United States have been treated using the OrbusNeich Teleport Microcatheter (Teleport; Cardiovascular Systems), which recently received US Food and...
TOBA II shows repairing dissections after angioplasty “clearly augments” outcomes for...
Thomas Zeller (Bad Krozingen, Germany) delves into data and design of the TOBA II (Tack Optimized Balloon Angioplasty II) trial with principal investigator William...
BD acquires TVA Medical, makers of everlinQ endoAVF System
BD (Becton, Dickinson and Company) announced it has completed the acquisition of TVA Medical, a company that develops minimally invasive vascular access solutions...
Medtronic receives US FDA clearance for 200mm and 250mm IN.PACT Admiral...
Medtronic has announced that it has received US FDA approval for 200mm and 250mm lengths of the IN.PACT Admiral drug-coated balloon (DCB) to treat...
Europe braces for harder times in medical device innovation while US...
Countries in the European Union have long been the first to receive new innovations in medical technology, as the EU’s Medical Device Directive (MDD)...
US FDA clears CorPath GRX system for use in peripheral vascular...
Corindus has announced receiving 510(k) clearance from the US Food and Drug Administration (FDA) for use of the CorPath GRX system in peripheral vascular...
LimFlow completes enrolment in US feasibility study
LimFlow has announced completion of enrolment of the original 10-patient cohort in the US feasibility study of the LimFlow percutaneous deep vein arterialisation system....
US FDA approves Gore Viabahn expandable stent graft for iliac artery...
The Gore Viabahn VBX balloon expandable endoprosthesis has received US Food & Drug Administration (FDA) approval for treatment of de novo or restenotic lesions found...
Robert Califf to resign as US FDA commissioner
Robert Califf is to step down as US Food and Drug Administration (FDA) commissioner upon the inauguration of Donald Trump as US President on...
US FDA bans powdered gloves
The US Food and Drug Administration (FDA) has issued a final rule to ban powdered surgical gloves, patient examination gloves and the absorbable powder...
NICE collaborates with US FDA on Payer Communication Taskforce
The UK’s National Institute for Health and Care Excellence (NICE) has announced its participation in the US Food and Drug Administration (FDA)’s Payer Communication...