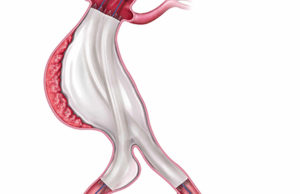
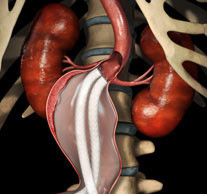
Tag: EVAS
Alexander Zimmermann
Alexander Zimmermann (University Hospital Zurich, Zurich, Switzerland) tells Vascular News about his career to date, beginning with a rotation in vascular surgery that opened...
VEITH 2021: Two-year follow-up data from EVAS2 IDE study delivered
The confirmatory EVAS2 clinical study to evaluate the safety and effectiveness of the Nellix endovascular aneurysm sealing system (EVAS; Endologix) for the treatment of...
Endologix receives FDA approval for chEVAS IDE study and signs distribution...
Endologix has announced the investigational device exemption (IDE) approval from the US Food and Drug Administration (FDA) to commence a new pivotal study to...
Marc Schermerhorn: Mortality analysis of EVAS vs. EVAR
Marc Schermerhorn (Boston, USA) discusses the three-year results of an analysis on the long-term survival of Nellix EVAS system (Endologix) IDE trial patients vs....
EVAS2 IDE clinical study enrols first patient
The first patient has been treated in the EVAS2 IDE confirmatory clinical study of the investigational Nellix EndoVascular Aneurysm Sealing (EVAS) System (Endologix) by...
Positive two-year Endologix Nellix clinical data presented at SVS 2017
Positive two-year clinical data from Endologix’ Nellix EVAS FORWARD investigative device exemption trial have been presented at the Society of Vascular Surgery (SVS; 31...
Personalising AAA Care With Polymer Sealing (OUS only)
In this supplement: -The clinical and biological advantages of active sac management with EVAS -10 things to know about Ovation -Ovation: Finally an endograft...
Endologix provides update on the Nellix sealing system US regulatory status
Endologix has met with the US Food and Drug Administration (FDA) regarding its Nellix endovascular aneurysm sealing system (EVAS System).
Based upon that meeting and...
Nellix IDE trial shows low morbidity and mortality with high procedural...
Primary safety and efficacy endpoints for the Nellix system (Endologix) investigational device exemption (IDE) trial have been achieved at one year, with “very low...