Tag: Aquedeon Medical
Aquedeon Medical receives US FDA approval to expand clinical trial of...
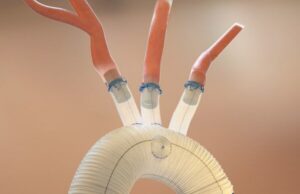
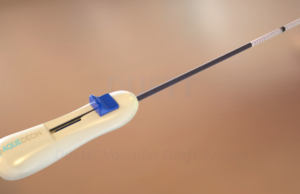
The US Food and Drug Administration (FDA) has approved the expansion of Aquedeon Medical's investigational device exemption (IDE) clinical trial evaluating the Duett vascular graft...
First patient enrolled in IDE study of Aquedeon Medical’s Duett vascular...
Aquedeon Medical has announced the initiation of an investigational device exemption (IDE) clinical trial to study the Duett vascular graft system.
A press release reveals...
Aquedeon Medical receives FDA IDE approval for the Duett vascular graft...
Aquedeon Medical has announced its receipt of US Food and Drug Administration (FDA) investigational device exemption (IDE) approval to conduct a staged pivotal clinical...