Shape Memory Medical recently announced that the first patient has been treated in the AAA-SHAPE pivotal trial.
AAA-SHAPE (Abdominal aortic aneurysm sac healing and prevention of expansion) is a prospective, multicentre, randomised, open-label trial to determine safety and effectiveness of the Impede-FX RapidFill device to improve abdominal aortic aneurysm (AAA) sac behaviour when used with elective endovascular aneurysm repair (EVAR).
The patient was treated by Raghu Motaganahalli (Indiana University School of Medicine, Indianapolis, USA).
“We would like to congratulate Dr Motaganahalli and the clinical study team at Indiana University for being the first to enrol a patient in the AAA-SHAPE pivotal trial. This is the first randomised controlled trial comparing EVAR plus sac management with Impede-FX RapidFill to standalone EVAR to evaluate shape memory polymer and its potential to improve sac regression in AAA patients,” said Ted Ruppel, president and chief executive officer of Shape Memory Medical.
AAA-SHAPE will enrol 180 patients with infrarenal AAA across up to 50 sites in the USA, Europe, and New Zealand. Study participants will be randomised 2:1, either to EVAR plus sac management with Impede-FX RapidFill (the treatment arm) or to standard EVAR (the control arm). Key endpoints will compare sac diameter and volume change, endoleak rates, and secondary interventions.
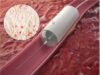
Impede-FX RapidFill, the investigational device, incorporates Shape Memory Medical’s novel shape memory polymer, a proprietary, porous, radiolucent, embolic scaffold that is crimped for catheter delivery and self-expands upon contact with blood. In AAA-SHAPE, Impede-FX RapidFill is intended to fill the aneurysm blood lumen around a commercially available EVAR stent graft to promote aneurysm thrombosis and sac shrinkage.
Prior to the AAA-SHAPE pivotal trial, the AAA-SHAPE early feasibility studies enrolled a combined 35 patients in New Zealand and The Netherlands.
“Large studies report that 60% of aneurysms either fail to regress or expand within one year following EVAR, a problem linked to rehospitalisations, secondary interventions, and increased mortality. Moving more patients toward sac regression has the potential for significant benefits,” said Marc Schermerhorn (Beth Israel Deaconess Medical Center, Boston, USA), AAA-SHAPE national principal investigator.
“We are pleased to be the first site to treat a patient within the AAA-SHAPE pivotal trial,” commented Motaganahalli, principal investigator. “The outcomes of this important trial will help determine whether Impede-FX RapidFill, with its unique properties, plays a meaningful role in post-EVAR AAA outcomes.”