The UK National Institute for Health and Care Excellence (NICE) has today published guidelines on abdominal aortic aneurysm (AAA) diagnosis and management. Discussion between physicians and patients to consider the risks of any intervention versus no intervention is encouraged, and, while the guidelines are in favour of open repair for most patients, they also highlight circumstances in which open repair is contraindicated because of structural or anaesthetic medical comorbidities.
When the draft guidelines were published in May 2018—which recommended that UK healthcare professionals should favour open repair over endovascular repair for unruptured aneurysms—they cast a shadow over EVAR and have since been the source of debate and controversy among the vascular community, which has been exacerbated by multiple publication delays.
These final recommendations indicate that NICE has listened to the physician community’s concerns to recognise that EVAR is sometimes the only option in certain patients. The new guidance places the onus upon the physician to discuss all the management options for unruptured aneurysms with the patient—conservative management, open surgical repair, and EVAR. It is also notable that there is a recommendation for following patients in the National Vascular Registry to learn more about results.
Furthermore, the responsibility now appears to be on physicians to discuss the known current shortcomings of EVAR with patients, which these final guidelines emphasise. EVAR has operative mortality and early mortality benefit for patients over open repair, but was found in trials to have inadequate return for follow-up. This meant that failing EVARs with endoleak and secondary rupture were not picked up with consequent unacceptably high AAA-related mortality, a finding that became more marked after eight years of follow-up.
Recommendations in focus
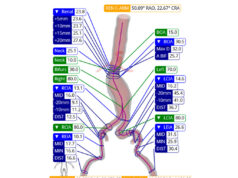
In the new section on elective repair of unruptured aneurysms (1.5), the NICE board states in recommendation 1.5.1 that aneurysm repair for patients with an unruptured AAA should be considered if it is symptomatic, asymptomatic, larger than 4cm and has grown by more than 1cm in one year (measured inner-to-inner maximum anterior-posterior aortic diameter on ultrasound), or asymptomatic and 5.5cm or more, but measured inner-to-inner. The guidelines here rely on the UK Small Aneurysm Trial (UK SAT) and ADAM trial that identified one of the criteria for intervention as growth of more than 1cm per year. Notably, however, they also use a 5.5cm inner-to-inner measurement in the recommendation. In the UK SAT, a smaller measurement was used—5.5cm outer-to-outer.
Recommendation 1.5.2 focuses on discussing the benefits and risks of repair or conservative management. It states: “When discussing aneurysm repair with patients who have an unruptured AAA, explain the overall balance of benefits and risks with repair and with conservative management, based on their current health and their expected future health. The decision on whether repair is preferred over conservative management should be made jointly by the patient and their clinician after assessment of a number of factors”. These include aneurysm size and morphology, the patient’s age, life expectancy, fitness for surgery, and other conditions they have, the risk of AAA rupture if they do not have repair, the short- and long-term benefits and risks, and other disadvantages of repair, and the uncertainties around estimates of risk for AAAs larger than 5.5cm, again measured inner-to-inner.
Recommendations 1.5.3 to 1.5.5 consider open surgical repair, standard EVAR, or conservative management. The guidelines suggest healthcare professionals offer open surgical repair for patients with unruptured AAAs “meeting the criteria in recommendation 1.5.1, unless it is contraindicated because of their abdominal copathology, anaesthetic risks, and/or medical comorbidities” (1.5.3), “who meet the criteria in recommendation 1.5.1 and who have an abdominal copathology […] that may make EVAR the preferred option” (1.5.4), and “meeting the criteria in recommendation 1.5.1 who have anaesthetic risks and/or medical comorbidities that would contraindicate open surgical repair” (1.5.5).
Finally, recommendations 1.5.6 and 1.5.7 outline complex EVAR diagnosis and management. If open surgical repair and complex EVAR are both suitable options (1.5.6), or for patients who have anaesthetic risks and/or medical comorbidities that would contraindicate open surgical repair (1.5.7), two factors should be taken into consideration. Firstly, only consider EVAR if the risks of complex EVAR compared with the risks of open surgical repair, and the uncertainties around whether complex EVAR improves perioperative survival or long-term outcome, when compared with open surgical repair, have been discussed. Secondly, only consider complex EVAR if it will be performed with special arrangements for consent and for audit and research that will determine the clinical and cost effectiveness of complex EVAR when compared with open surgical repair, and all patients are entered onto the National Vascular Registry.
Comment

Ian Loftus, consultant vascular surgeon at St George’s University Hospital NHS Foundation Trust (London, UK) and past president of the Vascular Society of Great Britain and Ireland (Vascular Society), spoke to Vascular News about the newly-published guidelines. He commented: “This has clearly been a very difficult process for NICE and for the professions involved as stakeholders. It would have been without precedent to see a recommendation to completely stop such an established therapy as endovascular aneurysm repair. The final version of the guidelines recognises the difficulties faced when a clinician sits with the individual patient, and the need for patient and clinician choice. It allows the flexibility to still proceed with endovascular repair in circumstances where that is felt to be an appropriate course of action.”
Loftus added: “This should be seen, however, as a wake-up call to endovascular practitioners and industry. We need to ensure that endovascular therapies are performed in appropriate patients, in an appropriate environment. Most importantly, we must work together to ensure long term durability of aneurysm repair. The collection of robust outcomes data, through the National Vascular Registry, is a vital part of the final guidelines.”

Sophie Renton, consultant vascular surgeon at London North West University Healthcare NHS Trust (London, UK) and secretary to the Vascular Society, also remarked on the recommendations: “I am pleased that the final NICE guidance recognises the importance of patient choice and gives greater autonomy to the doctor-patient relationship. I believe these recommendations are a pragmatic solution. The Vascular Society will be working to support implementation of the guidelines and monitor progress of implementation through the NVR.”

Michael Jenkins, consultant vascular surgeon at St Mary’s Hospital, Imperial College Healthcare NHS Foundation Trust (London, UK), commented: “Crucially the final NICE AAA guidance gives back autonomy to clinicians and takes into account patient choice and shared decision making on an individual patient basis. It continues to acknowledge the better durability of open repair, but now does accept the place of EVAR under certain circumstances including consideration of patient fitness.
“Additionally, the new guidance defines the place of custom devices and the importance of patient information during consent to clarify where we are uncertain regarding long term durability. Closer scrutiny and ongoing surveillance of such device performance over time within the UK National Vascular Registry is vital and will ultimately gives us longer term data to decide the future utility of these devices. Although anatomical suitability and use within manufacturer’s ‘instructions for use’ is not stated, it is implied that it will be an important criterion within MDT decision making.
“Importantly, allowing ongoing use of EVAR in controlled circumstances will allow us to re-evaluate where grafts perform well to help develop better and more durable devices for the future. It is now up to the vascular community to sensibly adopt and implement these guidelines and accept the important message behind them.”