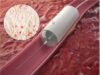
Results from Veryan’s Mimics randomised controlled trial have been published in the latest issue of Circulation: Cardiovascular Interventions. Use of the BioMimics 3D helical stent resulted in higher patency at two years when compared with a straight stent, for the treatment of symptomatic disease of the superficial femoral and popliteal arteries.
The Mimics trial is the first randomised trial to compare two bare-metal stents to treat the condition and demonstrate that stent design influences clinical outcome. According to a company release, the study demonstrates the benefits of the BioMimics stent design, which is designed to impart natural curvature to the diseased artery when implanted, to promote swirling blood flow with the aim of improving the outcome of peripheral intervention.
The 200th patient has been enrolled into Veryan’s MIMICS-2 study; a prospective, single-arm, multicentre clinical study of 280 patients at more than 40 investigational sites in the USA, Germany and Japan. The study is being conducted under a US Food and Drug Administration (FDA) Investigational Device Exemption (IDE), with Japanese PMDA concurrence through the Harmonization by Doing initiative, to provide clinical data to support parallel premarket approval reviews in US and Japan.
Thomas Zeller (Bad Krozingen, Germany), MIMICS-2 German national principal investigator, comments, “The outstanding effort and diligence of all investigators and research coordinators is making the MIMICS-2 Study the fastest-ever enrolling SFA stenting study of its type. There is a lot of commitment from everyone involved in this important study and we eagerly look forward to completing enrolment in the summer.”
A MIMICS-2 case was featured live via satellite at this month’s New Cardiovascular Horizons Annual Conference in New Orleans, USA.
Veryan is also anticipating the first patient being enrolled into the company-sponsored MIMICS-3D Registry. MIMICS-3D is a prospective, multicentre, observational registry to evaluate the BioMimics 3D self-expanding stent system in the treatment of peripheral arterial disease. The Registry will evaluate safety, effectiveness and device performance within a real-world clinical population in a minimum of 500 patients across Europe.