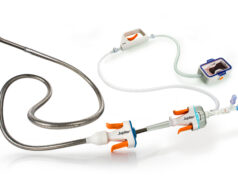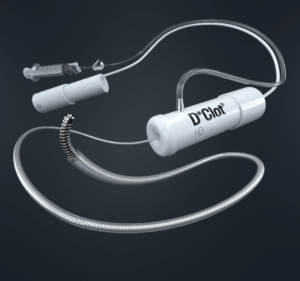
Mermaid Medical Group recently announced US commercial availability of the D-Clot HD rotational thrombectomy system. As of 12 January, the company reports 25 successful human cases.
‘‘I am extremely pleased to see dialysis patients who are heavily involved with the healthcare system benefits from the release of D-Clot HD.’’ said Lars Vinther, CEO of Mermaid Medical Group.
A multicentre limited market release evaluation demonstrated the system’s ability to restore flow effectively and safely with no reported complications. The data derives from several institutions where numerous users applied the technology to patients and showed effectiveness across variations in vessel size, access type, and thrombus morphology.
“Today marks a major milestone for the company as we are transitioning to full commercialisation of the product in the USA,’’ stated Lars Vinther. The D-Clot HD will provide an added mechanical thrombectomy option for physicians involved in arteriovenous (AV) access flow restoration.