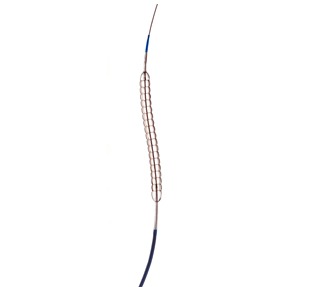
QT Vascular has signed a definitive agreement with Medtronic, for the worldwide distribution of its Chocolate percutaneous transluminal angioplasty (PTA) catheter for a period of five years and automatically renewable for two additional one-year periods. The parties continue to negotiate other aspects of their commercial relationship.
Chocolate’s nitinol-constraining structure is designed to provide atraumatic dilatation in the treatment of blocked arteries. The Chocolate BAR1 post-market registry of 490 patients showed that use of the device results in low rates of dissections and bailout stenting. Chocolate PTA may be used as a stand-alone treatment or adjunctive treatment for stenosis in vessels above and below the knee. It is approved for use in the USA, Europe, Australia, Turkey, Singapore and Hong Kong.
Current sales of the device in the USA are conducted through the direct sales force of the company’s wholly-owned subsidiary, TriReme Medical, and a distribution partner. Outside the USA, sales are conducted via country-specific distribution partners. Under the new distribution agreement with Medtronic, transition of the Chocolate PTA business will occur on a country-by-country basis over the coming months starting with the USA.
According to a company release, QT Vascular plans to focus on its drug-coated Chocolate program. The company hopes to have full IDE approval from the US Food and Drug Administration.













