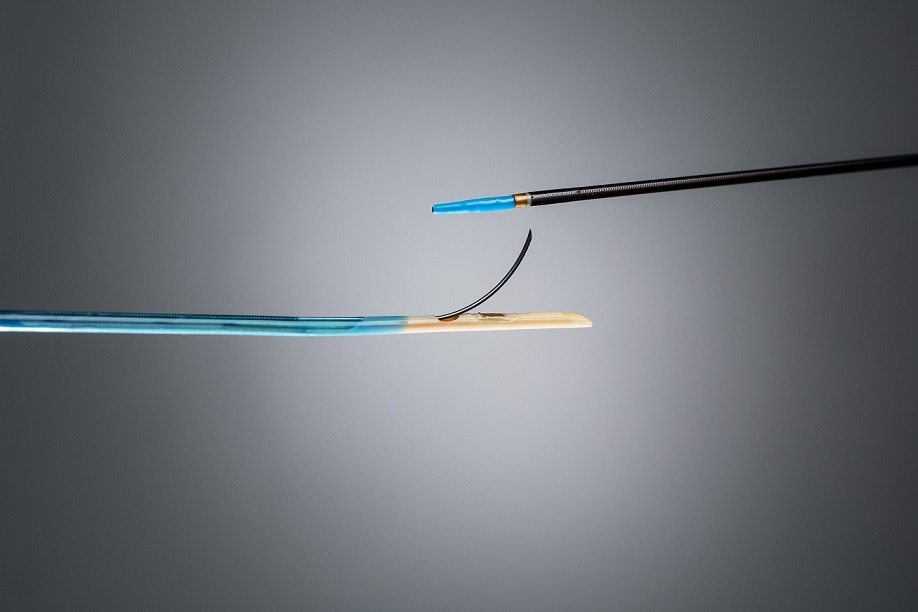
LimFlow has received CE mark for its fully percutaneous LimFlow system designed for venous arterialisation of the lower limbs in end-stage patients at risk of limb amputation for critical limb ischaemia.
The LimFlow system is a percutaneous therapy for patients who have exhausted all current revascularisation options. It is designed to promote chronic wound healing and avoid major amputation. It uses proprietary ultrasound guided catheters and covered nitinol stents to uniquely bypass diseased arteries and divert blood flow into the tibial vein in order to vascularise the entire ischemic foot.
“Due to the ongoing epidemic of diabetes and cardiovascular disease, every year more and more patients are presenting with end-stage critical limb ischaemia, and the tools we have to treat them are limited,” says Steven Kum, vascular surgeon at Changi Hospital in Singapore. “The LimFlow system is a new and critically important option for patients who suffer substantially from their ischemic foot. This therapy will create a strong foundation for us as vascular specialists, working with a wound care team, to provide new hope for them.”
“Utilising the existing alternative pathway of the venous vasculature, the LimFlow system is designed to reestablish perfusion for patients that have chronic, non-healing wounds and are in imminent danger of losing a limb,” says Dan Rose, chief executive officer of LimFlow. “We can now provide an option for patients that have none today. In early clinical cases, we have seen patients with extensive and severe foot wounds, including gangrene, fully heal following treatment with the LimFlow therapy, becoming mobile and active again.”
First-in-man and CE mark clinical studies have been completed for the LimFlow system. The initial clinical results have been presented at the Amputation Prevention Symposium (AMP) and the Vascular International Advances Annual Conference (VIVA), and journal publication of these results is expected soon.













