Laminate Medical Technologies has announced the close of a US$8m Series B financing round. This round of funding will help finance continued development and marketing of VasQ.
“The funding will enable us to expand our activities in the European market, where we are already working successfully with leading hospitals in the UK, Switzerland, Italy and Germany, as well as to complete clinical trials in the USA as we seek FDA approval,” said Laminate chief executive officer Tammy Gilon. “As part of this effort, we are currently expanding our staff of professionals experienced in medical devices and opening a branch of the company in the USA.”
Since its formation in 2012, total investment in Laminate is US$13m. A number of European funds took part in Series B, as did the Chinese pharmaceutical giant Haisco. Laminate’s existing investors are private investors, including Nava and Yehuda Zisapel, Zohar Gilon, Eri Steimatzky, Ari Raved and a grant from the chief scientist in Israel’s Ministry of the Economy.
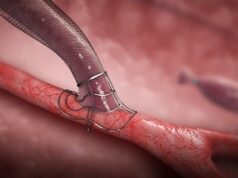
Developed by Laminate, VasQ is intended for patients suffering from kidney failure and in need of dialysis, which requires vascular access. The most common and preferred method of vascular access is an arteriovenous fistula, created by surgically suturing a blood vessel connection between an artery and a vein, usually in the region of the wrist or the elbow. Two intravenous needles are inserted through this connection to remove the patient’s blood for filtration in the dialysis machine, and then return it. This connection ensures a sufficiently high transfer of blood volume (veins do not transfer sufficient volume, while arteries are too deep for repeated insertion of a needle).
Unfortunately, a venous blockage forms in more than half of these cases. In response to increased pressure, the vein wall thickens, which in turn affects the blood flow during dialysis. As a result, the patient must have a repeat operation, affecting the ability to receive treatment and creating a burden on the hospital’s resources.
VasQ is a sleeve placed over the vein, creating an optimal geometric configuration with the artery and reducing the tension in the vein. This allows proper blood flow during dialysis while reducing venous thickening and blockage. Studies show VasQ has significant success.
“The technique for fistula surgery has not changed since it was first performed in 1966. Because it has a high failure rate, patients with kidney failure who are on dialysis have to repeat the fistula operation every few years – in many cases, after just a few months,” Gilon said. “VasQ is designed to prevent this traumatic cycle and allow patients to continue dialysis treatment uninterrupted.”
VasQ is in clinical trials for approval by the FDA. The CE marked device is already in use in hospitals in Europe and in Israel, with impressive results. Recently, dozens of hospitals across Germany have received funding to implement the VasQ after receiving NUB statues 1 approval.”