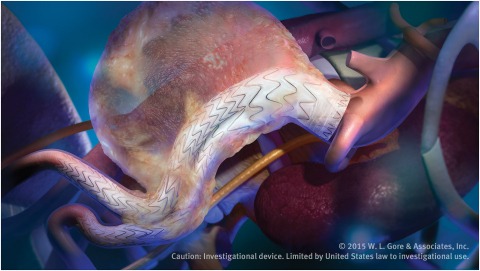
Gore has announced the first European patient implant of the Gore Excluder Conformable AAA Endoprosthesis with Active Control System. This next-generation endovascular aneurysm repair (EVAR) device is indicated to treat the broadest range of abdominal aortic aneurysms (AAA) in patients with challenging anatomies. Physicians now have an EVAR solution designed to treat patients previously excluded due to challenging proximal aortic necks.
The successful procedure took place on 11 September at Catharina Hospital in Eindhoven, the Netherlands by Marc van Sambeek. The patient was also the first enrolment in an investigator initiated post-market European registry, known as Excel (Excluder Conformable real life), of which van Sambeek is the principal investigator.
“Before this device, EVAR was reserved for patients whose aorta fell within a standard shape and size,” said van Sambeek. “The conformability of the new Gore Excluder Conformable device in combination with the angulation control of the new delivery system will allow us to offer a less invasive, lower-risk alternative to open surgery to more AAA patients than ever before. The Excel registry will track the real-world effectiveness and safety of the device, and I look forward to enrolling additional patients with challenging, highly angulated aortic anatomies and seeing the long-term value of this device.”
The Excel registry will enrol 150 patients from up to 11 European sites. Data from the registry will assess safety and treatment success of the Gore Excluder Conformable AAA device for the treatment of infrarenal AAA in a broad range of anatomic presentations. The device is also currently being evaluated in a pivotal investigational US study, in which the first patient was enrolled on 19 December, 2017.
The Gore Excluder Conformable AAA device builds on the proven clinical performance of the Gore Excluder AAA Endoprosthesis, which represents over 20 years of worldwide experience and more than 300,000 patients treated, a history unmatched by currently available AAA stent grafts. The new device leverages the limb design of the Gore Excluder device which has demonstrated exceptional clinical performance as evidenced by 0.5% limb occlusion through three-year follow-up. The limbs, a unique combination of proprietary ePTFE graft material and a fully supported, nested, nitinol stent, are designed to prevent kinking and occlusion.
The new device introduces the Gore Active Control System in AAA treatment. This new delivery system includes a conformable stent graft, enhanced device positioning, and optional angulation control. To enhance positioning, the device is deployed partially constrained and the physician has the option to reconstrain the proximal end of the device to aide in achieving optimal device placement. During the deployment process, the physician may use the optional angulation control to angle the device to achieve orthogonal placement to the aortic blood flow lumen and to maximise device conformability and seal.
“With this first implant in Europe, we are continuing our commitment to offer the broadest and most technically advanced endovascular treatment capabilities to physicians and their patients,” said Eric Zacharias, vascular business leader at Gore. “We are dedicated to developing technology that enables physicians to better treat challenging aortic anatomies. By collaborating with physicians, we’ve evolved our market-leading Gore Excluder device to enable more patients to be treated with EVAR.”