Endologix has announced the 24-month results of the DETOUR2 study, presented at VAM 2023 (14–17 June, National Harbor, USA), the annual meeting of the Society for Vascular Surgery (SVS), by one of the study’s principal investigators, Sean Lyden (Cleveland Clinic, Cleveland, USA).
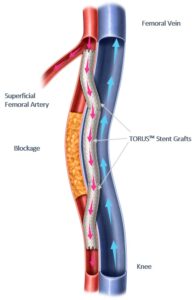
Percutaneous transmural arterial bypass (PTAB) with the Detour System, recently received premarket approval Approval from the US Food and Drug Administration (FDA). The system enables physicians to percutaneously bypass lesions in the superficial femoral artery, by using stents routed through the femoral vein to restore blood flow to the leg. The Detour system is comprised of the Endocross device and Torus stent grafts.
The DETOUR2 study enrolled 202 patients in the USA and Europe. The 24-month results from the study indicated that 96% of enrolled patients had chronic total occlusions, with a mean lesion length of 32.7cm. Technical success was achieved in 100% of treated patients and the primary safety endpoint was surpassed with a 30-day major adverse event (MAE) rate of 7%
The freedom from clinically driven target lesion revascularisation (CD-TLR) at 24 months was 76.7%, and secondary patency was 82.3%. The freedom from symptomatic deep vein thrombosis (DVT) was 96.5%, and freedom from major lower limb amputation was 98.5% at 24 months.
“The two-year results from the DETOUR2 Study are encouraging and demonstrate PTAB using the Detour system offers good patency rates in long superficial femoral artery (SFA) lesions. As noted in the conclusion of the presentation, the two-year data mimics those of surgical bypass without the need for general anaesthesia, long length of stay, and high risk of complications. We look forward to continuing to study the DETOUR System,” said Lyden.
“We are delighted to present the two-year results of the DETOUR2 Study which investigates the use of the PTAB therapy in patients with very long SFA lesions,” said Matt Thompson, president and CEO of Endologix. “The results suggest that the DETOUR System offers a viable approach in patients where open surgery is the currently recommended treatment. We are excited to see more patients benefit from this unique approach to the treatment of complex PAD.”














Merci beaucoup pour toutes ces informations.
Cordialement