Contego Medical has completed enrolment in the Paladin Carotid Post-dilation Balloon System Registry in Europe. The registry involves 5 centres with 100 total patients, and represents the first prospective clinical evaluation of a new class of device that provides Integrated Embolic Protection (IEP) technology for interventional treatment of carotid stenosis.
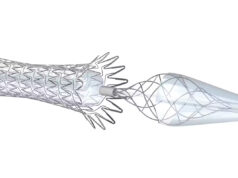
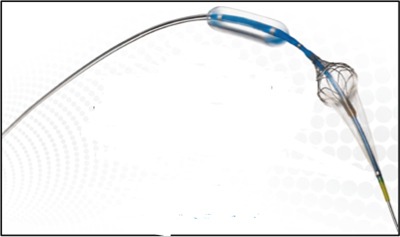
The Paladin system offers improved outcomes by reducing the risk of embolisation during post-dilation, when the patient has the highest risk of embolic events that can lead to stroke. The single-step system integrates a post-dilation balloon proximally and a small-particle embolic protection filter distally at the catheter tip. A company press release says that the “intuitive and versatile system enhances a physician’s ability to safely treat carotid stenosis.”
“As principle investigator of this study, it is gratifying to see a new type of technology available that addresses safer treatment of patients with carotid artery disease,” said Thomas Zeller, head of Angiology at Universitäts-Herzzentrum Freiburg in Bad Krozingen, Germany. “I am encouraged by our results with Paladin and look forward to using devices with IEP technology for other cardiovascular applications.”
“This is a very important study in demonstrating the importance of small particle embolisation capture for carotid stenting,” said Ralf Langhoff, director of Angiology, Saint Getrauden Hospital, Berlin, Germany. “Procedural success rate has been high and interim results of 30-day magnetic resonance imaging scans are very promising. I am pleased to be part of this registry and the study of a new technology that can reduce embolisation and stroke incidence for these procedures to almost zero without adding any sophisticated and time-consuming procedural steps. The technology is already embedded into our standard-of-care procedure technique for carotid artery stenting.”