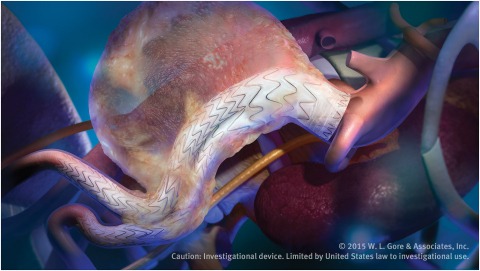
Gore has received CE mark for its Excluder conformable abdominal aortic aneurysm (AAA) device, a product designed for the treatment of abdominal aortic aneurysms in patients with challenging anatomies.
The device incorporates stent design elements similar to the Conformable Gore Tag device, intended to enable apposition in tortuous aortas. Approved for patients with either proximal aortic neck angles of up to 90 degrees or a minimum aortic neck length of 10 mm, the product is designed to allow for tailored treatment and precision in more difficult anatomies.
“Before this approval, in order to qualify for EVAR, the patient had to fit a fairly narrow subset of anatomical criteria,” says Robert Rhee, chief of Vascular and Endovascular Surgery at Maimonides Medical Center (New York City, USA). “The Gore Excluder conformable AAA endoprosthesis will expand the applicability of EVAR to patients traditionally considered to have anatomy too challenging for endovascular treatment.”
The device also introduces Gore’s Active Control system. This technology is intended to allow physicians to angle the device to optimise conformability and maximise seal in the proximal aortic neck.













