BTG has announced it has acquired Novate Medical, a medical device company focused on the prevention of pulmonary embolism in patients at high risk of venous thromboembolic events.
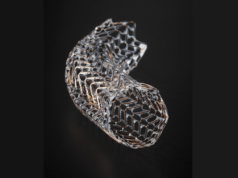
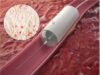
Novate has developed Sentry, the first bioconvertible inferior vena cava filter, which has recently been granted 510(k) regulatory clearance in the USA. Sentry’s 12 month clinical trial data demonstrated no new symptomatic pulmonary embolism and no evidence of device migration, tilt, fracture, perforation or embolization, complications which have been associated with some other inferior vena cava filters. The unique bioconversion feature eliminates the need for an additional interventional procedure to retrieve the device.
BTG plans to launch Sentry in the US in the second half of FY2018/19 and will sell the device through its existing vascular sales force.
“This bolt-on acquisition further enhances BTG’s strength in the vascular space.” said Louise Makin, BTG’s CEO.
“Novate’s unique inferior vena cava filter offers our existing customers a highly complementary product in the management of pulmonary embolism.”
BTG paid US$20m in cash to acquire Novate and may be required to pay additional cash considerations up to US$130m if certain commercial and sales-related milestones are met.