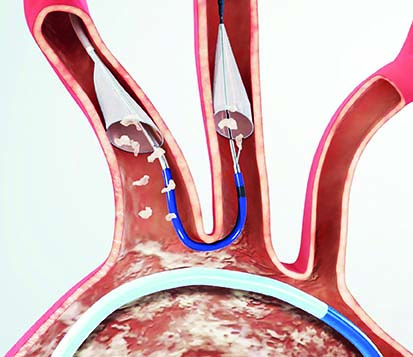
 Boston Scientific has announced that it has recently closed its acquisition of Claret Medical, which developed and commercialised the Sentinel cerebral embolic protection system—the only device cleared in the USA and Europe to protect patients against the risk of stroke in transcatheter aortic valve implantation (TAVI) procedures.
Boston Scientific has announced that it has recently closed its acquisition of Claret Medical, which developed and commercialised the Sentinel cerebral embolic protection system—the only device cleared in the USA and Europe to protect patients against the risk of stroke in transcatheter aortic valve implantation (TAVI) procedures.
The company also announced that the US Centers for Medicare and Medicaid Services (CMS) granted a New Technology Add-on Payment (NTAP) designation for the Sentinel system as part of the federal fiscal year 2019 Inpatient Prospective Payment System (IPPS). The NTAP designation, awarded to new medical devices determined to substantially improve the diagnosis or treatment of Medicare beneficiaries, will be effective on October 1, 2018.
Kevin Ballinger, president, Interventional Cardiology, Boston Scientific, comments: “The Sentinel System is an exciting platform technology designed to reduce the risk of procedure-related stroke in TAVI and other left-heart and endovascular procedures, and is an increasingly important consideration for patients and physicians as the TAVI indication expands to treat a younger patient population. The recent CMS NTAP designation underscores the clinical value of the Sentinel System and will allow for accelerated adoption of this adjunctive therapy amongst structural heart centres.”
Boston Scientific announced a definitive agreement to acquire Claret Medical on July 20, 2018 for $220 million in up-front cash with an additional $50 million payment for reaching a reimbursement-based milestone, which has been fulfilled with the recent NTAP designation.