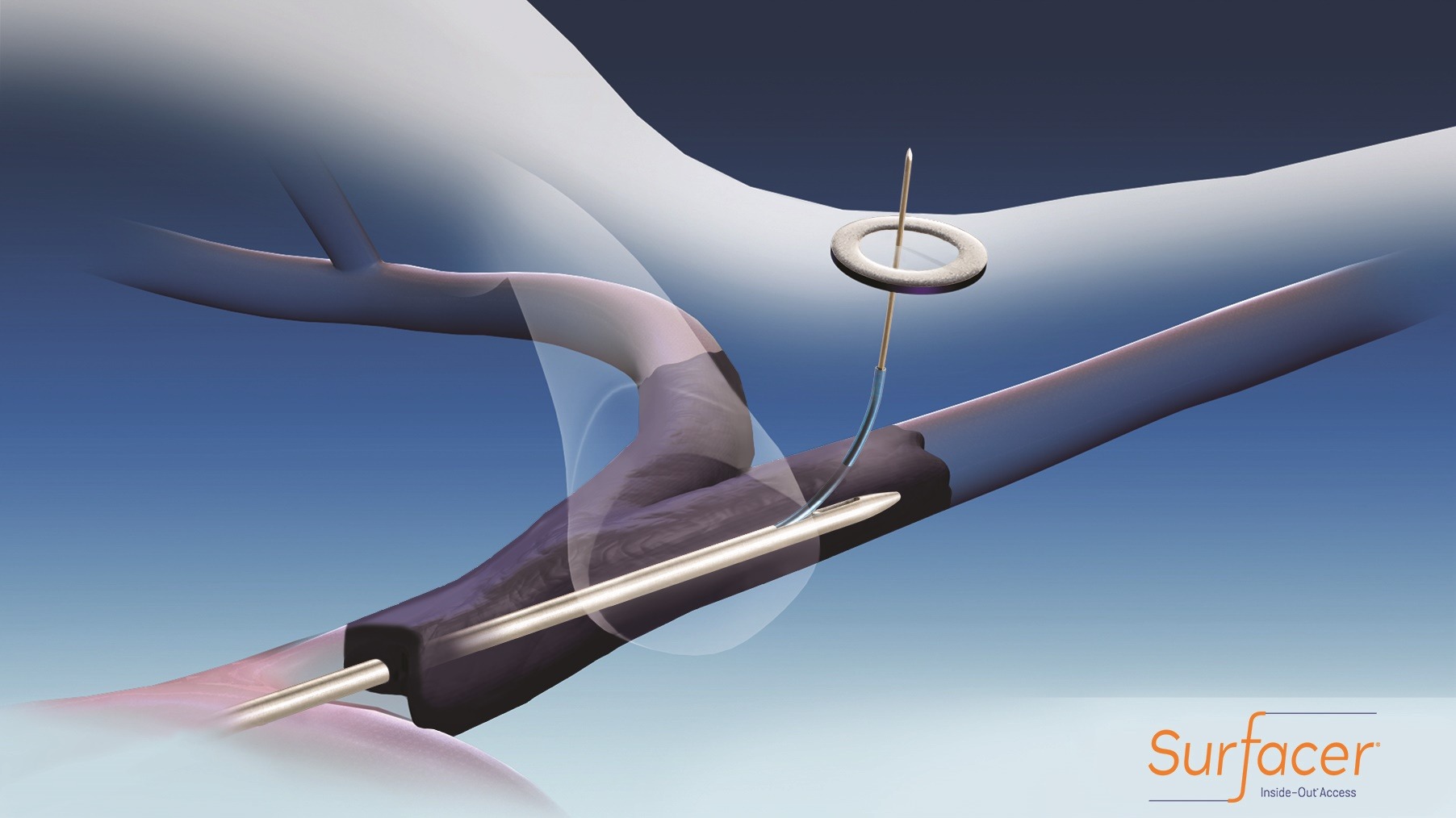
The Surfacer Inside-Out Access Catheter System (Bluegrass Vascular) has demonstrated positive commercial use, consistently achieving central venous access in patients with upper body occlusions. A study presented in a poster session at the 2018 American Society of Nephrology (ASN) Annual Meeting (23–28 October, San Diego, USA) builds on positive results from the company’s post-market international SAVE Registry announced earlier this month.
The results of the retrospective, independent study evaluating 32 cases with the Surfacer System demonstrated an impressive 97% success rate in all patients. In one patient access was not achieved due to significant scoliosis altering the anatomy. There were zero device-related complications reported, including bleeding, haematoma and catheter-related infection, and all patients displayed similar catheter function at three months.
“The clinical application of the Surfacer System, as shown in this study, proves to be extremely positive,” states Roman Reindel-Schwaighofer, Nephrology and Dialysis Fellow at the Medical University of Vienna in Austria and the lead author of the study. “The Surfacer System provides a safe and effective solution that both preserves and restores vascular access for patients requiring haemodialysis who otherwise have very limited options.”
The Surfacer System is a CE-marked device designed to reliably, safely and repeatedly gain central venous access for haemodialysis patients awaiting maturation of permanent vascular access. Failed venous access attempts may prevent permanent arteriovenous access, increasing patient morbidity and the overall cost of care.
“Based on these results, I am very optimistic about the clinical impact of the Surfacer System and its ability to treat upper body vascular occlusions,” states Gürkan Sengölge, associate professor of Medicine, Nephrology and Intensive Care Medicine at the Medical University of Vienna and the study’s senior author. “The ability to safely perform the procedure, in an outpatient setting, and have the option to repeat when necessary, has the potential to change the standard of care going forward.”
“This is an exciting study that mirrors the commercial successes recently revealed in our international post-market SAVE Registry and similar positive trends observed in our SAVE-US IDE study,” states Gabriele Niederauer, CEO and president of Bluegrass Vascular. “I look forward to announcing additional data as it becomes available and as we near completion of our SAVE-US IDE trial.”











