This article forms part of an educational supplement sponsored by Shockwave Medical. Explore the full Compliance is Key series here.

Stefano Fazzini (Tor Vergata Hospital, Rome, Italy) goes into the details and the data behind Shockwave Intravascular Lithotripsy (IVL; Shockwave Medical)—a technology he states is “changing the boundaries” in peripheral arterial disease (PAD).
Are we still confined by the complexity of calcium when treating vascular disease? Endovascular treatment is a front-line therapy for patients with PAD and the presence of heavy calcification represents the most hostile anatomical challenge we face as vascular specialists.
A poor vessel preparation can lead to dissections and high residual stenosis and consequently increased utilisation of stents. Stenting outcomes can also be significantly affected by calcium with poor stent expansion leading to acute/early failure through stent thrombosis and/or crush. Drug uptake with a drug-coated balloon (DCB) has also been proven to be reduced by the barrier of calcium.1
As disease becomes more complex, the need for effective and consistent vessel preparation is important and this can facilitate improved outcomes and a leave nothing behind strategy. This approach has become a reality, and specialty percutaneous transluminal angioplasty (PTA) or debulking devices can provide a solution.2
One of the most innovative technologies available today is Shockwave IVL. IVL can significantly improve effectiveness at the vessel preparation phase.
Peripheral arteries differ from coronary arteries, which have a high level of elastic fibres; they are predominantly muscular and affected with medial calcification and consequent loss of vessel compliance. Together with increasing luminal gain and minimising complications, a better vessel compliance is the key to a superior vessel prep with IVL and the novelty that differentiates it from other techniques.
Calcific plaques impair vascular distensibility and need high pressures to be modified, leading to complications such as flow-limiting dissections, embolisation, or life-threatening complications such as a rupture (e.g. in the iliac arteries).
The changing paradigm of IVL uses shockwaves delivered on an intuitive low-pressure (4 ATM) balloon platform to avoid vessel barotrauma. This unique mechanism of action derives from a miniaturisation of shockwave treatment used in the treatment of kidney stones for over 30 years with adaptations made for safety and effectiveness, and is easy to use to modify cardiovascular calcium. The IVL source, in contrast to that for extracorporeal lithotripsy, has close proximity to the calcium and the goal is only to fracture, not pulverise.

These shockwaves are a specific form of sonic pressure waves produced by emitters arrayed within a balloon angioplasty catheter. A small electrical discharge at the emitters vaporises the fluid and creates a rapidly expanding bubble within the balloon.
Shockwaves fracture superficial and deep calcium with an effective pressure of ~50 ATM, changing vessel compliance and allowing an improved luminal gain, while safely passing through the soft tissue; fractures occur when shockwaves encounter tissue with higher acoustic impedance, like calcium, while they have no effect on soft tissue due to having similar impedance to water..
One of the most appealing safety features is the lower risks of embolisation because calcium remains in situ, it is disrupted and not pulverised, with the internal elastic lamina acting as a membrane.3
Compared to the use of atherectomy, IVL is not a complex and time-consuming procedure, but requires a few simple indications for use to improve effectiveness, such as a proper sizing. It should be 10% oversized compared to the reference vessel diameter.4
Despite being a relatively new tool, IVL has a growing data pool to back up the science with a randomised controlled trial (RCT) that demonstrated superiority in procedural success vs. conventional PTA in femoropopliteal lesions, with a reduction in complications and 75% reduction in the need for provisional stenting.4
The PAD III RCT demonstrated superior vessel preparation in an IVL group compared to a PTA group, with no complications (embolisations, perforations, thrombus, no flow), atraumatic treatment (max ballooning pressure: 6.3 ATM vs. 11.3 ATM, p<0.0001), reduced dissections (type ≥C dissections: 3.5% vs. 15.1%, p=0.03) and bailout stenting (4.6% vs. 18.3%, p=0.0002).4
William A Gray and Gunnar Tepe, co-principal investigators of DISRUPT PAD III, recently published two-year outcomes that confirm previous successful data: primary patency favoured IVL over PTA at one year (80.5% vs. 68%; p=0.017) and remained favourable through two years (74.4% vs. 57.7%; p=0.005).5
The PAD III RCT was reinforced by the PAD III observational study (OS)—a large all-comers registry that showed IVL to be an effective tool across all peripheral vessel beds, achieving consistently low residual stenoses and extremely low complications.6
The Disrupt PAD III OS with 1,373 patients and 1,677 lesions represents the largest prospective ‘real-world’ evidence for the treatment of heavily calcified PAD, and showed consistent outcomes with the randomised trial: an exceptional safety profile without final angiographic complications and proven effective calcium modification (residual final stenosis of 22% vs. 25%, PAD III RCT vs. OS, respectively).6,7
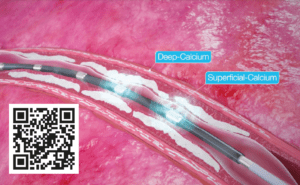
These optimal outcomes from the PAD III OS reinforce the predictability of IVL and its ability to consistently modify calcium across multiple peripheral vessel beds (iliac, femoral, popliteal and infrapopliteal arteries), challenging lesions (severe calcification, chronic total occlusions, long [>15cm] and eccentric lesions) and complex patients (chronic limb-threatening ischaemia, dialysis, and female patients).
The safety characteristics of IVL mean it is an effective tool in territories that have previously been difficult to treat with debulking technologies. Due to the low rates of dissections and distal emboli and no need for embolic protection devices, bifurcation disease can be simplified. The reduction in bailout stenting that has been observed also favours the use of IVL as vessel prep in ‘high-flexion zones’ and territory considered ‘no-stent zones’; in these districts, stents historically have not performed well when calcium is a contributing factor.
In the iliac district, IVL can open new pathways to become an adjunctive treatment for both occlusive (reducing kissing stent, stent length, covered stent, stent recoil) and aortic disease to facilitate hostile calcified access for large-bore devices (reducing iliac ruptures and use of open/endoconduits).
Recently, we presented at the 2022 European Society for Vascular Surgery (ESVS) annual meeting (20–23 September, Rome, Italy) our IVL experience to facilitate aortic endograft delivery in heavily calcified iliac access.8 There are two main indications, access only and associated iliac occlusive disease, in which an increased vessel compliance and luminal gain play a key role.
A third indication for using IVL prior to aortic endografts is allowing proper iliac limbs expansion. Heavily calcified iliac arteries could pose an obstacle to the correct expansion of iliac extension, resulting in an increased risk for limb occlusion. Even in this case, an accurate vessel preparation with IVL could prevent this risk, avoiding adjunctive stenting inside the iliac limbs.
The goal of changing vessel compliance aims to restore natural mechanics to the vessel, adding elasticity and increasing pulsatility. This is a paradigm shift in how we should approach calcium. Compliance is key and opens the door to successful treatments, changing previous procedure plans and preserving many future options.
Duplex ultrasonography could be a potential intraoperative adjunctive imaging tool to assess the effects of endovascular revascularisation in patients with peripheral calcified lesions. A better vessel compliance is well detected by a better Doppler waveform (monophasic changing to multiphasic) and duplex can confirm results from angiograms.
IVL is emerging as a powerful tool for the treatment of heavily calcified peripheral arteries and enabling new vascular pathways. It is changing the boundaries in PAD and transfemoral access for large-bore devices.
References
- Fanelli F, Cannavale A, Gazzetti M, et al. Calcium burden assessment and impact on drug-eluting balloons in peripheral arterial disease. Cardiovasc Intervent Radiol. 2014;37(4):898– 907.
- Ormiston W, Dyer-Hartnett S, Fernando R, et al. An update on vessel preparation in lower limb arterial intervention. CVIR Endovascular. 2020 Nov 27;3(1):86.
- Kereiakes DJ, Virmani R, Hokama JY, et al. Principles of intravascular lithotripsy for calcific plaque modification. JACC: Cardiovascular Interventions. 2021;14(12):1275–92.
- Tepe G, Brodmann M, Werner M, et al. Intravascular lithotripsy from the randomised Disrupt PAD III trial. JACC: Cardiovascular Interventions. 2021;14(12)1352–61.
- Tepe G, Brodmann M, Bachinsky W, et al. Intravascular lithotripsy for peripheral artery calcification: mid-term outcomes from the randomized Disrupt PAD III trial. Journal of the Society for Cardiovascular Angiography & Interventions. 2021 Jun 28;14(12):1352–1361.
- Adams G, Soukas PA, Mehrle A, et al. Intravascular lithotripsy for treatment of calcified infrapopliteal lesions: results from the Disrupt PAD III observational study. J Endovasc Ther. 2022 Feb;29(1):76–83.
- Armstrong EJ, Soukas PA, Sammas N, et al. Intravascular lithotripsy for treatment of calcified, stenotic iliac arteries: a cohort analysis from the Disrupt PAD III study. Cardiovasc Revasc Med. 2020 Oct;21(10):1262–1268.
- Fazzini S, Bosiers M, et al. A paradigm shift in tackling hostile calcified access. ESVS annual meeting, Rome, 2022 Sep.
Stefano Fazzini is a consultant of vascular surgery at Tor Vergata Hospital in Rome, Italy.













